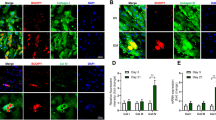
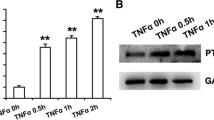
Abstract
Regenerative medicine and tissue engineering have delivered new healing possibilities to the treatment of soft tissue defects, but the selection of seed cells is critical for treatment. Adipose-derived stem cells have perpetually been a preferred candidate for seed cells due to their wealthy sources, simple access, high plasticity, and powerful value-added capabilities. How to improve the efficiency of adipogenic differentiation is the key to the treatment. Pituitary adenylate cyclase-activating peptide, as a biologically active peptide secreted by the pituitary, is widely involved in regulating the body’s sugar metabolism and lipid metabolism. However, the effects of MPAPO in ADSCs adipogenic differentiation remain unknown. Our results reveal that MPAPO treatment improves the adipogenic differentiation efficiency of ADSCs, including promoting the accumulation of lipid droplets and triglycerides, and the expression of adipocyte protein biomarkers PPARγ and C/EBPa. Additionally, the mechanism studies showed that the effective window of MPAPO-induced adipogenesis was the first 3 days during ADSCs differentiation. MPAPO selectively binds to the PAC1 receptor and promotes adipogenic differentiation of ADSCs by activating the ERK signaling pathway and elevating cell proliferation during postconfluent mitosis stage. Altogether, we demonstrate that MPAPO plays a crucial role in ADSCs adipogenesis, providing experimental basis and data for exploring therapeutic options in tissue defect repair.
Graphical Abstract








Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from every author upon request.
References
Zuk, P. A., Zhu, M., Mizuno, H., Huang, J., Futrell, J. W., Katz, A. J., et al. (2001). Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering, 7, 211–228.
Gimble, J., & Guilak, F. (2003). Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy, 5, 362–369.
Raposio, E., Bertozzi, N., Bonomini, S., Bernuzzi, G., Formentini, A., Grignaffini, E., et al. (2016). Adipose-derived Stem Cells Added to Platelet-rich Plasma for Chronic Skin Ulcer Therapy. Wounds, 28, 126–131.
Caruana, G., Bertozzi, N., Boschi, E., Pio Grieco, M., Grignaffini, E., & Raposio, E. (2015). Role of adipose-derived stem cells in chronic cutaneous wound healing. Annali Italiani di Chirurgia, 86, 1–4.
Murphy, J. M., Fink, D. J., Hunziker, E. B., & Barry, F. P. (2003). Stem cell therapy in a caprine model of osteoarthritis. Arthritis and Rheumatism, 48, 3464–3474.
Argentati, C., Morena, F., Bazzucchi, M., Armentano, I., Emiliani, C., Martino, S. (2018). Adipose Stem Cell Translational Applications: From Bench-to-Bedside. International journal of molecular sciences, 19, 3475.
Feisst, V., Meidinger, S., & Locke, M. B. (2015). From bench to bedside: use of human adipose-derived stem cells. Stem Cells Cloning, 8, 149–162.
Morena, F., Argentati, C., Calzoni, E., Cordellini, M., Emiliani, C., D’Angelo, F., et al. (2016). Ex-vivo tissues engineering modeling for reconstructive surgery using human adult adipose stem cells and polymeric nanostructured matrix. Nanomaterials (Basel), 6, 57.
Rosen, E. D., & Spiegelman, B. M. (2006). Adipocytes as regulators of energy balance and glucose homeostasis. Nature, 444, 847–853.
Lee, J. E., Schmidt, H., Lai, B., & Ge, K. (2019). Transcriptional and epigenomic regulation of adipogenesis. Molecular and Cellular Biology, 39, e00601–00618.
Ross, S. E., Hemati, N., Longo, K. A., Bennett, C. N., Lucas, P. C., Erickson, R. L., et al. (2000). Inhibition of adipogenesis by Wnt signaling. Science, 289, 950–953.
Lagathu, C., Christodoulides, C., Tan, C. Y., Virtue, S., Laudes, M., Campbell, M., et al. (2010). Secreted frizzled-related protein 1 regulates adipose tissue expansion and is dysregulated in severe obesity. International Journal of Obesity, 34, 1695–1705.
Vujovic, S., Henderson, S. R., Flanagan, A., & Clements, M. O. (2007). Inhibition of γ-secretases alters both proliferation and differentiation of mesenchymal stem cells. Cell Proliferation, 40, 185–195.
Song, B., Chi, Y., Li, X., Du, W., Han, Z. B., Tian, J., et al. (2015). Inhibition of Notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cellular Physiology and Biochemistry, 36, 1991–2002.
Bowers, R. R., & Lane, M. D. (2007). A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle, 6, 385–389.
Bowers, R. R., Kim, J. W., Otto, T. C., & Lane, M. D. (2006). Stable stem cell commitment to the adipocyte lineage by inhibition of DNA methylation: role of the BMP-4 gene. Proceedings of the National Academy of Sciences, 103, 13022–13027.
James, A. W. (2013). Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica (Cairo), 2013, 684736.
Prusty, D., Park, B. H., Davis, K. E., & Farmer, S. R. (2002). Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. Journal of Biological Chemistry, 277, 46226–46232.
Patel, A. L., & Shvartsman, S. Y. (2018). Outstanding questions in developmental ERK signaling. Development, 145, dev143818.
Asrih, M., Mach, F., Nencioni, A., Dallegri, F., Quercioli, A., & Montecucco, F. (2013). Role of mitogen-activated protein kinase pathways in multifactorial adverse cardiac remodeling associated with metabolic syndrome. Mediators of Inflammation, 2013, 367245.
Bost, F., Aouadi, M., Caron, L., Even, P., Belmonte, N., Prot, M., et al. (2005). The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes, 54, 402–411.
Ling, H. Y., Wen, G. B., Feng, S. D., Tuo, Q. H., & Liao, D. F. (2011). MicroRNA-375 promotes 3T3-L1 adipocyte differentiation through modulation of extracellular signal-regulated kinase signaling. Clinical & Experimental Pharmacology & Physiology, 38, 239.
Kwak, D. H., Lee, J. H., Kim, T., Ahn, H. S., Cho, W. K., Ha, H., et al. (2012). Aristolochia manshuriensis Kom inhibits adipocyte differentiation by regulation of ERK1/2 and Akt pathway. PLoS One1, 7, e49530.
Miyata, A., Arimura, A., Dahl, R. R., Minamino, N., Uehara, A., Jiang, L., et al. (1989). Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochemical And Biophysical Research Communications, 164, 567–574.
Shen, S., Gehlert, D. R., & Collier, D. A. (2013). PACAP and PAC1 receptor in brain development and behavior. Neuropeptides, 47, 421–430.
Rudecki, A. P., & Gray, S. L. (2016). PACAP in the defense of energy homeostasis. Trends in Endocrinology and Metabolism, 27, 620–632.
Li, M., Maderdrut, J. L., Lertora, J. J., & Batuman, V. (2007). Intravenous infusion of pituitary adenylate cyclase-activating polypeptide (PACAP) in a patient with multiple myeloma and myeloma kidney: a case study. Peptides, 28, 1891–1895.
Zhu, L., Tamvakopoulos, C., Xie, D., Dragovic, J., Shen, X., Fenyk-Melody, J. E., et al. (2003). The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: in vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1–38). Journal of Biological Chemistry, 278, 22418–22423.
Ma, Y., Zhao, S., Wang, X., Shen, S., Ma, M., Xu, W., et al. (2015). A new recombinant PACAP-derived peptide efficiently promotes corneal wound repairing and lacrimal secretion. Investigative Ophthalmology & Visual Science, 56, 4336–4349.
Adams, B. A., Gray, S. L., Isaac, E. R., Bianco, A. C., Vidal-Puig, A. J., & Sherwood, N. M. (2008). Feeding and metabolism in mice lacking pituitary adenylate cyclase-activating polypeptide. Endocrinology, 149, 1571–1580.
Arsenijevic, T., Gregoire, F., Chiadak, J., Courtequisse, E., Bolaky, N., Perret, J., et al. (2013). Pituitary adenylate cyclase activating peptide (PACAP) participates in adipogenesis by activating ERK signaling pathway. PLoS One, 8, e72607.
Ramírez-Zacarías, J. L., Castro-Muñozledo, F., & Kuri-Harcuch, W. (1992). Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry, 97, 493–497.
Chen, Y., Ikeda, K., Yoneshiro, T., Scaramozza, A., Tajima, K., Wang, Q., et al. (2019). Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature, 565, 180–185.
Wilfling, F., Haas, J. T., Walther, T. C., & Farese, R. V., Jr. (2014). Lipid droplet biogenesis. Current Opinion in Cell Biology, 29, 39–45.
Tang, W., Zeve, D., Suh, J. M., Bosnakovski, D., Kyba, M., Hammer, R. E., et al. (2008). White fat progenitor cells reside in the adipose vasculature. Science, 322, 583–586.
Cristancho, A. G., & Lazar, M. A. (2011). Forming functional fat: a growing understanding of adipocyte differentiation. Nature Reviews Molecular Cell Biology, 12, 722–734.
Tatsuno, I., Uchida, D., Tanaka, T., Saeki, N., Hirai, A., Saito, Y., et al. (2001). Maxadilan specifically interacts with PAC1 receptor, which is a dominant form of PACAP/VIP family receptors in cultured rat cortical neurons. Brain Research, 889, 138–148.
Ruiz-Ojeda, F. J., Rupérez, A. I., Gomez-Llorente, C., Gil, A., & Aguilera, C. M. (2016). Cell models and their application for studying adipogenic differentiation in relation to obesity: a review. International Journal of Molecular Sciences, 17, 1040.
Siersbæk, R., Nielsen, R., & Mandrup, S. (2012). Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends in Endocrinology & Metabolism, 23, 56–64.
Ntambi, J. M., & Young-Cheul, K. (2000). Adipocyte differentiation and gene expression. The Journal of Nutrition, 130, 3122S-3126S.
Lee, J. S., Ha, L., Park, J. H., & Lim, J. Y. (2012). Mechanical stretch suppresses BMP4 induction of stem cell adipogenesis via upregulating ERK but not through downregulating Smad or p38. Biochemical and Biophysical Research Communications, 418, 278–283.
Jin, Y., Zhang, W., Liu, Y., Zhang, M., Xu, L., Wu, Q., et al. (2014). rhPDGF-BB via ERK pathway osteogenesis and adipogenesis balancing in ADSCs for critical-sized calvarial defect repair. Tissue Engineering Part A, 20, 3303–3313.
Mandl, M., Wagner, S. A., Hatzmann, F. M., Mitterberger-Vogt, M. C., Zwierzina, M. E., Mattesich, M., et al. (2019). Sprouty1 is a weight-loss target gene in human adipose stem/progenitor cells that is mandatory for the initiation of adipogenesis. Cell Death & Disease, 10, 1–10.
Boney, C. M., Gruppuso, P. A., Faris, R. A., & Frackelton, A. R. The critical role of Shc in insulin-like growth factor-I-mediated mitogenesis and differentiation in 3T3-L1 preadipocytes. Molecular Endocrinology, 14, 805–813.
Morris, E. J., Jha, S., Restaino, C. R., Dayananth, P., Zhu, H., Cooper, A., et al. (2013). Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discovery, 3, 742–750.
Lindén, A., Hansson, L., Andersson, A., Palmqvist, M., Arvidsson, P., Löfdahl, C. G., et al. (2003). Bronchodilation by an inhaled VPAC(2) receptor agonist in patients with stable asthma. Thorax, 58, 217–221.
Zuk, P. A. (2010). The adipose-derived stem cell: looking back and looking ahead. Molecular Biology Of The Cell, 21, 1783–1787.
Young, D. A., Choi, Y. S., Engler, A. J., & Christman, K. L. (2013). Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials, 34, 8581–8588.
Li, F., Wang, D., Zhou, Y., Zhou, B., Yang, Y., Chen, H., et al. (2008). Protein kinase A suppresses the differentiation of 3T3-L1 preadipocytes. Cell Research, 18, 311–323.
Guo, X., Yu, R., Xu, Y., Lian, R., Yu, Y., Cui, Z., et al. (2016). PAC1R agonist maxadilan enhances hADSC viability and neural differentiation potential. Journal of Cellular And Molecular Medicine, 20, 874–890.
Yokota, C., Kawai, K., Ohashi, S., Watanabe, Y., & Yamashita, K. (1995). PACAP stimulates glucose output from the perfused rat liver. Peptides, 16, 55–60.
Gray, S. L., Cummings, K. J., Jirik, F. R., & Sherwood, N. M. (2001). Targeted disruption of the pituitary adenylate cyclase-activating polypeptide gene results in early postnatal death associated with dysfunction of lipid and carbohydrate metabolism. Molecular Endocrinology, 15, 1739–1747.
Siersbæk, R., Nielsen, R., & Mandrup, S. (2012). Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends in Endocrinology and Metabolism, 23, 56–64.
Ntambi, J. M., & Young-Cheul, K. (2000). Adipocyte differentiation and gene expression. Journal of Nutrition, 130, 3122s–3126s.
Sun, Z., Yang, X., Liu, Q. S., Li, C., Zhou, Q., Fiedler, H., et al. (2019). Butylated hydroxyanisole isomers induce distinct adipogenesis in 3T3-L1 cells. Journal of Hazardous Materials, 379, 120794.
Scott, R. E., Florine, D. L., Wille, J. J., Jr., & Yun, K. (1982). Coupling of growth arrest and differentiation at a distinct state in the G1 phase of the cell cycle: GD. Proceedings of the National Academy of Sciences of the United States of America, 79, 845–849.
Jang, Y. J., Koo, H. J., Sohn, E. H., Kang, S. C., Rhee, D. K., & Pyo, S. (2015). Theobromine inhibits differentiation of 3T3-L1 cells during the early stage of adipogenesis via AMPK and MAPK signaling pathways. Food & Function, 6, 2365–2374.
Umek, R. M., & Friedman, A., D. (1991). CCAAT-enhancer binding protein: A component of a differentiation switch. Science, 251, 288–292.
Chen, K., He, H., Xie, Y., Zhao, L., Zhao, S., Wan, X., et al. (2015). miR-125a-3p and miR-483-5p promote adipogenesis via suppressing the RhoA/ROCK1/ERK1/2 pathway in multiple symmetric lipomatosis. Scientific Reports, 5, 11909.
Kusuyama, J., Komorizono, A., Bandow, K., Ohnishi, T., & Matsuguchi, T. (2016). CXCL3 positively regulates adipogenic differentiation. Journal of Lipid Research, 57, 1806–1820.
Bost, F., Aouadi, M., Caron, L., Even, P., Belmonte, N., Prot, M., et al. (2005). The Extracellular Signal-Regulated Kinase Isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes, 54, P402-411.
Acknowledgements
I would like to thank all the students in the lab for their help and the members of the research group for their efforts. I would also like to thank for the funding supports of the National Natural Science Foundation of China (No. 82073748 and 81741130); the Guang-dong Basic and Applied Basic Research Foundation (No. 2019A1515011866 and 2021A1515010993); Guang-dong Science and Technology Innovation Strategy Special fund (International science and technology cooperation projects, No. 2021A0505030034).
Funding
This study was supported by the National Natural Science Foundation of China (No. 82073748 and 81741130); the Guang-dong Basic and Applied Basic Research Foundation (No. 2019A1515011866 and 2021A1515010993); Guang-dong Science and Technology Innovation Strategy Special fund (International science and technology cooperation projects, No. 2021A0505030034).
Author information
Authors and Affiliations
Contributions
Zixian Wang and Yi Ma contributed to the conception; Zixian Wang and Jianmin Liu carried out the experiments; Zixian Wang, Jianmin Liu, Yongmei Huang, Qian Liu, Meng Chen, and Yi Ma contributed to data or analysis tools and performed the analysis; Zixian Wang, Jianmin Liu, Qian Liu, Chunyan Ji, Meng Chen, and Yi Ma reviewed the articles and wrote the original draft; Zixian Wang, Yongmei Huang, Jia Feng, and Yi Ma contributed to the manuscript revision and editing; All the authors read and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Ethics Approval
Not applicable.
Consent to Participate
All authors agreed to participate in the study of the subject.
Consent for Publication
All authors agree to revise the paper for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOC 2.20 MB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Z., Liu, J., Huang, Y. et al. Pituitary Adenylate Cyclase-activating Polypeptide (PACAP) -derived Peptide MPAPO Stimulates Adipogenic Differentiation by Regulating the Early Stage of Adipogenesis and ERK Signaling Pathway. Stem Cell Rev and Rep 19, 516–530 (2023). https://doi.org/10.1007/s12015-022-10415-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10415-y