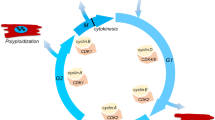
Abstract
Cardiac organoids (COs) are miniaturized and simplified organ structures that can be used in heart development biology, drug screening, disease modeling, and regenerative medicine. This cardiac organoid (CO) model is revolutionizing our perspective on answering major cardiac physiology and pathology issues. Recently, many research groups have reported various methods for modeling the heart in vitro. However, there are differences in methodologies and concepts. In this review, we discuss the recent advances in cardiac organoid technologies derived from human embryonic stem cells (hESCs) and human-induced pluripotent stem cells (hiPSCs), with a focus on the summary of methods for organoid generation. In addition, we introduce CO applications in modeling heart development and cardiovascular diseases and discuss the prospects for and common challenges of CO that still need to be addressed. A detailed understanding of the development of CO will help us design better methods, explore and expand its application in the cardiovascular field.
Graphical abstract




Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- CFS:
-
Cardiac fibroblasts
- COs:
-
Cardiac organoids
- CMs:
-
Cardiomyocytes
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- CX43:
-
Connexin 43
- cAMP:
-
Cyclic AMP
- dECMs:
-
Decellularized ECMs
- DMD:
-
Duchenne muscular dystrophy
- EB:
-
Embryoid body
- ECM:
-
Extracellular matrix
- EHM:
-
Engineered human myocardium
- EAT:
-
Engineered cardiac tissues
- FHF:
-
First heart field
- HFOs:
-
Heart-forming organoids
- hCOs:
-
Human cardiac organoids
- hESCs:
-
Human-embryonic stem cells
- hiPSCs:
-
Human-induced pluripotent stem cells
- MTs:
-
Microtissues
- ReWs:
-
Reentrant waves
- SHF:
-
Second heart field
- VLs:
-
Vessel-like structures
References
Zwi-Dantsis, L., & Gepstein, L. (2012). Induced pluripotent stem cells for cardiac repair [J]. Cellular and Molecular Life Sciences, 69(19), 3285–3299.
Kim, J., Koo, B. K., & Knoblich, J. A. (2020). Human organoids: model systems for human biology and medicine [J]. Nature Reviews Molecular Cell Biology, 21(10), 571–584.
McCauley, H. A., & Wells, J. M. (2017). Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish [J]. Development, 144(6), 958–962.
Voges, H. K., Mills, R. J., Elliott, D. A., et al. (2017). Development of a human cardiac organoid injury model reveals innate regenerative potential [J]. Development, 144(6), 1118–1127.
Fatehullah, A., Tan, S. H., & Barker, N. (2016). Organoids as an in vitro model of human development and disease [J]. Nature Cell Biology, 18(3), 246–254.
Correia, C., Koshkin, A., Duarte, P., et al. (2018). 3D aggregate culture improves metabolic maturation of human pluripotent stem cell derived cardiomyocytes [J]. Biotechnology and Bioengineering, 115(3), 630–644.
Chen, V. C., Ye, J., Shukla, P., et al. (2015). Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells [J]. Stem Cell Research, 15(2), 365–375.
Kempf, H., Kropp, C., Olmer, R., et al. (2015). Cardiac differentiation of human pluripotent stem cells in scalable suspension culture [J]. Nature Protocols, 10(9), 1345–1361.
Domian, I. J., Chiravuri, M., van der Meer, P., et al. (2009). Generation of functional ventricular heart muscle from mouse ventricular progenitor cells [J]. Science, 326(5951), 426–429.
Kattman, S. J., Witty, A. D., Gagliardi, M., et al. (2011). Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines [J]. Cell Stem Cell, 8(2), 228–240.
Eschenhagen, T., & Zimmermann, W. H. (2005). Engineering myocardial tissue [J]. Circulation Research, 97(12), 1220–1231.
Beauchamp, P., Moritz, W., Kelm, J. M., et al. (2015). Development and Characterization of a Scaffold-Free 3D Spheroid Model of Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes [J]. Tissue Engineering. Part C, Methods, 21(8), 852–861.
Polonchuk, L., Chabria, M., Badi, L., et al. (2017). Cardiac spheroids as promising in vitro models to study the human heart microenvironment [J]. Science and Reports, 7(1), 7005.
Daly, A. C., Davidson, M. D., & Burdick, J. A. (2021). 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels [J]. Nature Communications, 12(1), 753.
Giacomelli, E., Meraviglia, V., Campostrini, G., et al. (2020). Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease [J]. Cell Stem Cell, 26(6), 862-879 e811.
Beauchamp, P., Jackson, C. B., Ozhathil, L. C., et al. (2020). 3D Co-culture of hiPSC-Derived Cardiomyocytes With Cardiac Fibroblasts Improves Tissue-Like Features of Cardiac Spheroids [J]. Frontiers in Molecular Biosciences, 7, 14.
Zuppinger, C. (2019). Measurement of Contractility and Calcium Release in Cardiac Spheroids [J]. Methods in Molecular Biology, 1929, 41–52.
Arai, K., Murata, D., Verissimo, A. R., et al. (2018). Fabrication of scaffold-free tubular cardiac constructs using a Bio-3D printer [J]. PLoS ONE, 13(12), e0209162.
Ong, C. S., Pitaktong, I., & Hibino, N. (2020). Principles of Spheroid Preparation for Creation of 3D Cardiac Tissue Using Biomaterial-Free Bioprinting [J]. Methods in Molecular Biology, 2140, 183–197.
Noguchi, R., Nakayama, K., Itoh, M., et al. (2016). Development of a three-dimensional pre-vascularized scaffold-free contractile cardiac patch for treating heart disease [J]. Journal of Heart and Lung Transplantation, 35(1), 137–145.
Pitaktong, I., Lui, C., Lowenthal, J., et al. (2020). Early Vascular Cells Improve Microvascularization Within 3D Cardiac Spheroids [J]. Tissue Engineering. Part C, Methods, 26(2), 80–90.
Caspi, O., Lesman, A., Basevitch, Y., et al. (2007). Tissue engineering of vascularized cardiac muscle from human embryonic stem cells [J]. Circulation Research, 100(2), 263–272.
Saini, H., Navaei, A., van Putten, A., et al. (2015). 3D cardiac microtissues encapsulated with the co-culture of cardiomyocytes and cardiac fibroblasts [J]. Adv Healthc Mater, 4(13), 1961–1971.
Radisic, M., Park, H., Martens, T. P., et al. (2008). Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue [J]. Journal of Biomedical Materials Research. Part A, 86(3), 713–724.
Li, Y., Asfour, H., & Bursac, N. (2017). Age-dependent functional crosstalk between cardiac fibroblasts and cardiomyocytes in a 3D engineered cardiac tissue [J]. Acta Biomaterialia, 55, 120–130.
Garzoni, L. R., Rossi, M. I., de Barros, A. P., et al. (2009). Dissecting coronary angiogenesis: 3D co-culture of cardiomyocytes with endothelial or mesenchymal cells [J]. Experimental Cell Research, 315(19), 3406–3418.
Burridge, P. W., Matsa, E., Shukla, P., et al. (2014). Chemically defined generation of human cardiomyocytes [J]. Nature Methods, 11(8), 855–860.
Pei, F., Jiang, J., Bai, S., et al. (2017). Chemical-defined and albumin-free generation of human atrial and ventricular myocytes from human pluripotent stem cells [J]. Stem Cell Research, 19, 94–103.
Zhao, Y., Rafatian, N., Feric, N. T., et al. (2019). A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling [J]. Cell, 176(4), 913-927 e918.
Oh, Y., Cho, G. S., Li, Z., et al. (2016). Functional Coupling with Cardiac Muscle Promotes Maturation of hPSC-Derived Sympathetic Neurons [J]. Cell Stem Cell, 19(1), 95–106.
Winbo, A., Ramanan, S., Eugster, E., et al. (2020). Functional coculture of sympathetic neurons and cardiomyocytes derived from human-induced pluripotent stem cells [J]. American Journal of Physiology. Heart and Circulatory Physiology, 319(5), H927–H937.
Bejleri, D., & Davis, M. E. (2019). Decellularized Extracellular Matrix Materials for Cardiac Repair and Regeneration [J]. Adv Healthc Mater, 8(5), e1801217.
Guyette, J. P., Charest, J. M., Mills, R. W., et al. (2016). Bioengineering Human Myocardium on Native Extracellular Matrix [J]. Circulation Research, 118(1), 56–72.
McCrary, M. W., Bousalis, D., Mobini, S., et al. (2020). Decellularized tissues as platforms for in vitro modeling of healthy and diseased tissues [J]. Acta Biomaterialia, 111, 1–19.
Basara Gozde, Ozcebe S. Gulberk, Ellis Bradley W., et al. (2021). Tunable human myocardium derived decellularized extracellular matrix for 3D bioprinting and cardiac tissue engineering [J]. Gels, 7(2), 70
Saludas, L., Pascual-Gil, S., Prosper, F., et al. (2017). Hydrogel based approaches for cardiac tissue engineering [J]. International Journal of Pharmaceutics, 523(2), 454–475.
Shkumatov, A., Baek, K., & Kong, H. (2014). Matrix rigidity-modulated cardiovascular organoid formation from embryoid bodies [J]. PLoS ONE, 9(4), e94764.
Asti, A., & Gioglio, L. (2014). Natural and synthetic biodegradable polymers: Different scaffolds for cell expansion and tissue formation [J]. International Journal of Artificial Organs, 37(3), 187–205.
Depalma, S. J., Davidson, C. D., Stis, A. E., et al. (2021). Microenvironmental determinants of organized iPSC-cardiomyocyte tissues on synthetic fibrous matrices [J]. Biomaterials Science, 9(1), 93–107.
Hendrickson, T., Mancino, C., Whitney, L., et al. (2021). Mimicking cardiac tissue complexity through physical cues: A review on cardiac tissue engineering approaches [J]. Nanomedicine, 33, 102367.
Leslie-Barbick, J. E., Saik, J. E., Gould, D. J., et al. (2011). The promotion of microvasculature formation in poly(ethylene glycol) diacrylate hydrogels by an immobilized VEGF-mimetic peptide [J]. Biomaterials, 32(25), 5782–5789.
Schneider, M. C., Chu, S., Randolph, M. A., et al. (2019). An in vitro and in vivo comparison of cartilage growth in chondrocyte-laden matrix metalloproteinase-sensitive poly(ethylene glycol) hydrogels with localized transforming growth factor beta3 [J]. Acta Biomaterialia, 93, 97–110.
Prakash Parthiban, S., Rana, D., Jabbari, E., et al. (2017). Covalently immobilized VEGF-mimicking peptide with gelatin methacrylate enhances microvascularization of endothelial cells [J]. Acta Biomaterialia, 51, 330–340.
Liu, N., Ye, X., Yao, B., et al. (2021). Advances in 3D bioprinting technology for cardiac tissue engineering and regeneration [J]. Bioact Mater, 6(5), 1388–1401.
Lee, A., Hudson, A. R., Shiwarski, D. J., et al. (2019). 3D bioprinting of collagen to rebuild components of the human heart [J]. Science, 365(6452), 482–487.
Kupfer, M. E., Lin, W. H., Ravikumar, V., et al. (2020). In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid [J]. Circulation Research, 127(2), 207–224.
Zuppinger, C. (2016). 3D culture for cardiac cells [J]. Biochimica et Biophysica Acta, 1863(7 Pt B), 1873–1881.
Shadrin, I.Y., Allen, B.W., Qian, Y., et al. (2017). Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues [J]. Nature Communications, 8(1), 1825.
Jackman, C. P., Ganapathi, A. M., Asfour, H., et al. (2018). Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation [J]. Biomaterials, 159, 48–58.
Gao, L., Gregorich, Z. R., Zhu, W., et al. (2018). Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine [J]. Circulation, 137(16), 1712–1730.
Hansen, A., Eder, A., Bonstrup, M., et al. (2010). Development of a drug screening platform based on engineered heart tissue [J]. Circulation Research, 107(1), 35–44.
Li, J., Zhang, L., Yu, L., et al. (2020). Circulating re-entrant waves promote maturation of hiPSC-derived cardiomyocytes in self-organized tissue ring [J]. Communications Biology, 3(1), 122.
Seguret, M., Vermersch, E., Jouve, C., et al. (2021). Cardiac organoids to model and heal heart failure and cardiomyopathies [J]. Biomedicines, 9(5), 563.
MacQueen, L. A., Sheehy, S. P., Chantre, C. O., et al. (2018). A tissue-engineered scale model of the heart ventricle [J]. Nature Biomedical Engineering, 2(12), 930–941.
Li, R. A., Keung, W., Cashman, T. J., et al. (2018). Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells [J]. Biomaterials, 163, 116–127.
Ho Beatrice Xuan, Sheng Pang Jeremy Kah, Phua Qian Hua, et al. (2021). Generation of human chambered cardiac organoids from pluripotent stem cells for improved modelling of cardiovascular diseases [J]. BioRxiv. https://doi.org/10.1101/2021.05.21.445153
Silva, A. C., Matthys, O. B., Joy, D. A., et al. (2021). Co-emergence of cardiac and gut tissues promotes cardiomyocyte maturation within human iPSC-derived organoids [J]. Cell stem cell, 28(12), 2137–2152.e6.
Rossi, G., Broguiere, N., Miyamoto, M., et al. (2021). Capturing Cardiogenesis in Gastruloids [J]. Cell Stem Cell, 28(2), 230-240 e236.
Drakhlis, L., Biswanath, S., Farr, C. M., et al. (2021). Human heart-forming organoids recapitulate early heart and foregut development [J]. Nature Biotechnology, 39(6), 737–746.
Richards, D. J., Li, Y., Kerr, C. M., et al. (2020). Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity [J]. Nature Biomedical Engineering, 4(4), 446–462.
Tiburcy, M., Hudson, J. E., Balfanz, P., et al. (2017). Defined Engineered Human Myocardium With Advanced Maturation for Applications in Heart Failure Modeling and Repair [J]. Circulation, 135(19), 1832–1847.
Sabater-Molina, M., Perez-Sanchez, I., Hernandez del Rincon, J. P., et al. (2018). Genetics of hypertrophic cardiomyopathy: A review of current state [J]. Clinical Genetics, 93(1), 3–14.
Ware, J. S., & Cook, S. A. (2018). Role of titin in cardiomyopathy: from DNA variants to patient stratification [J]. Nature Reviews Cardiology, 15(4), 241–252.
Ohiri, J. C., & McNally, E. M. (2018). Gene Editing and Gene-Based Therapeutics for Cardiomyopathies [J]. Heart Failure Clinics, 14(2), 179–188.
Chen, Q., Kirsch, G. E., Zhang, D., et al. (1998). Genetic basis and molecular mechanism for idiopathic ventricular fibrillation [J]. Nature, 392(6673), 293–296.
Arbelo, E., Sarquella-Brugada, G., & Brugada, J. (2016). Gene-Specific Therapy for Congenital Long QT Syndrome: Are We There Yet? [J]. Journal of the American College of Cardiology, 67(9), 1059–1061.
Knott, G. J., & Doudna, J. A. (2018). CRISPR-Cas guides the future of genetic engineering [J]. Science, 361(6405), 866–869.
Chadwick, A. C., & Musunuru, K. (2017). Genome Editing for the Study of Cardiovascular Diseases [J]. Current Cardiology Reports, 19(3), 22.
Guilinger, J. P., Thompson, D. B., & Liu, D. R. (2014). Fusion of catalytically inactive Cas9 to FokI nuclease improves the specificity of genome modification [J]. Nature Biotechnology, 32(6), 577–582.
Slaymaker, I. M., Gao, L., Zetsche, B., et al. (2016). Rationally engineered Cas9 nucleases with improved specificity [J]. Science, 351(6268), 84–88.
Kleinstiver, B. P., Pattanayak, V., Prew, M. S., et al. (2016). High-fidelity CRISPR-Cas9 nucleases with no detectable genome-wide off-target effects [J]. Nature, 529(7587), 490–495.
Chen, J. S., Dagdas, Y. S., Kleinstiver, B. P., et al. (2017). Enhanced proofreading governs CRISPR-Cas9 targeting accuracy [J]. Nature, 550(7676), 407–410.
Schwank, G., Koo, B. K., Sasselli, V., et al. (2013). Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients [J]. Cell Stem Cell, 13(6), 653–658.
Kodo, K., Ong, S. G., Jahanbani, F., et al. (2016). iPSC-derived cardiomyocytes reveal abnormal TGF-beta signalling in left ventricular non-compaction cardiomyopathy [J]. Nature Cell Biology, 18(10), 1031–1042.
Mosqueira, D., Mannhardt, I., Bhagwan, J. R., et al. (2018). CRISPR/Cas9 editing in human pluripotent stem cell-cardiomyocytes highlights arrhythmias, hypocontractility, and energy depletion as potential therapeutic targets for hypertrophic cardiomyopathy [J]. European Heart Journal, 39(43), 3879–3892.
Ceholski, D. K., Turnbull, I. C., Kong, C. W., et al. (2018). Functional and transcriptomic insights into pathogenesis of R9C phospholamban mutation using human induced pluripotent stem cell-derived cardiomyocytes [J]. Journal of Molecular and Cellular Cardiology, 119, 147–154.
Lan, F., Lee, A. S., Liang, P., et al. (2013). Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells [J]. Cell Stem Cell, 12(1), 101–113.
Limpitikul, W. B., Dick, I. E., Tester, D. J., et al. (2017). A Precision Medicine Approach to the Rescue of Function on Malignant Calmodulinopathic Long-QT Syndrome [J]. Circulation Research, 120(1), 39–48.
Gahwiler, E. K. N., Motta, S. E., Martin, M., et al. (2021). Human iPSCs and Genome Editing Technologies for Precision Cardiovascular Tissue Engineering [J]. Frontiers in Cell and Developmental Biology, 9, 639699.
Long, C., Li, H., Tiburcy, M., et al. (2018). Correction of diverse muscular dystrophy mutations in human engineered heart muscle by single-site genome editing [J]. Science Advances, 4(1), eaap9004.
Yang, K. C., Breitbart, A., de Lange, W. J., et al. (2018). Novel Adult-Onset Systolic Cardiomyopathy Due to MYH7 E848G Mutation in Patient-Derived Induced Pluripotent Stem Cells [J]. JACC: Basic to Translational Science, 3(6), 728–740.
Feng Wei, Schriever Hannah, Jiang Shan, et al. (2020). Computational profiling of hiPSC-derived heart organoids reveals chamber defects associated with Ebstein’s anomaly [J]. BioRxiv. https://doi.org/10.1101/2020.12.24.424346
Driehuis, E., & Clevers, H. (2017). CRISPR/Cas 9 genome editing and its applications in organoids [J]. American Journal of Physiology. Gastrointestinal and Liver Physiology, 312(3), G257–G265.
Wilke, R. A., Lin, D. W., Roden, D. M., et al. (2007). Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges [J]. Nature Reviews Drug Discovery, 6(11), 904–916.
Mills, R. J., Parker, B. L., Quaife-Ryan, G. A., et al. (2019). Drug Screening in Human PSC-Cardiac Organoids Identifies Pro-proliferative Compounds Acting via the Mevalonate Pathway [J]. Cell Stem Cell, 24(6), 895-907 e896.
Kitsuka, T., Itoh, M., Amamoto, S., et al. (2019). 2-Cl-C.OXT-A stimulates contraction through the suppression of phosphodiesterase activity in human induced pluripotent stem cell-derived cardiac organoids [J]. PLoS One, 14(7), e0213114.
Skardal, A., Aleman, J., Forsythe, S., et al. (2020). Drug compound screening in single and integrated multi-organoid body-on-a-chip systems [J]. Biofabrication, 12(2), 025017.
Rajan, S. A. P., Aleman, J., Wan, M., et al. (2020). Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform [J]. Acta Biomater, 106, 124–135.
Yin, F., Zhang, X., Wang, L., et al. (2021). HiPSC-derived multi-organoids-on-chip system for safety assessment of antidepressant drugs [J]. Lab on a Chip, 21(3), 571–581.
Nie, Y. Z., Zheng, Y. W., Ogawa, M., et al. (2018). Human liver organoids generated with single donor-derived multiple cells rescue mice from acute liver failure [J]. Stem Cell Research & Therapy, 9(1), 5.
Feric, N. T., Pallotta, I., Singh, R., et al. (2019). Engineered cardiac tissues generated in the Biowire™ II: a platform for human-based drug discovery [J]. Toxicological Sciences : an Official Journal of the Society of Toxicology, 172(1), 89–97.
Acknowledgements
We would like to thank Professor Wang Li from Fuwai Hospital for his training and support.
Funding
The author’s work is supported by Xiamen Science and Technology Plan Project Grant (No. 3502Z20209138), National Natural Science Foundation of China Youth Foud (No. 82100441).
Author information
Authors and Affiliations
Contributions
Liyuan Zhu performed data analysis and interpretation, and wrote the manuscript. Kui Lui and Qi Feng assisted with data collection and provided feedback. Yingnan Liao assembled the data, carried out the data analysis and interpretation, wrote the manuscript, and gave final approval of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, L., Liu, K., Feng, Q. et al. Cardiac Organoids: A 3D Technology for Modeling Heart Development and Disease. Stem Cell Rev and Rep 18, 2593–2605 (2022). https://doi.org/10.1007/s12015-022-10385-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10385-1