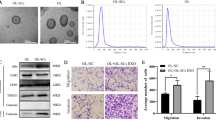
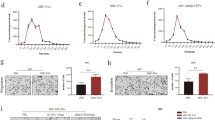
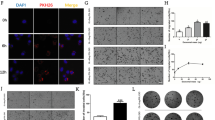
Abstract
High mortality rate and poor survival in melanoma are associated with efficient metastatic colonization. The underlying mechanisms remain elusive. Elucidating the role of exosomes in mediating the interactions between cancer cells and the metastatic microenvironment has been focused on cancer cell derived exosomes in modulating the functions of stromal cells. Whether cancer stem cells (CSCs) can modify the metastatic properties of non-CSC cells, and whether exosomal crosstalk plays a role have not been demonstrated prior to this report. In this study, a paired M14 melanoma derivative cell line, i.e., melanoma parental cell (MPC) and its CSC derivative cell line melanoma stem cell (MSC) were employed. We demonstrated that exosomal crosstalk betwen MSCs and non-CSC MPCs is a new mechanism that underlies melanoma metastasis. Low metastatic melanoma cells (MPCs) can acquire the “metastatic power” from highly metastatic melanoma CSCs (MSCs). We illustrated an uncharacterized microRNA, miR-4535 in mediating such exosomal crosstalk. MSCs deliver its exosomal miR-4535 to the targeted MPCs. Upon entering MPCs, miR-4535 augments metastatic colonization of MPCs by inactivating the autophagy pathway.
Graphical Abstract






Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from every author upon request.
References
Gensbittel, V., et al. (2021). Mechanical adaptability of tumor cells in metastasis. Developmental Cell, 56(2), 164–179.
Schadendorf, D., et al. (2018). Melanoma. The Lancet, 392(10151), 971–984.
Batlle, E., & Clevers, H. (2017). Cancer stem cells revisited. Nature Medicine, 23(10), 1124–1134.
Regan, J. L., et al. (2017). Non-canonical hedgehog signaling is a positive regulator of the WNT pathway and is required for the survival of Colon Cancer stem cells. Cell Reports, 21(10), 2813–2828.
Lee, S. Y., et al. (2017). Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Molecular Cancer, 16(1), 1–25.
Hu, J., et al. (2017). A CD44v(+) subpopulation of breast cancer stem-like cells with enhanced lung metastasis capacity. Cell Death & Disease, 8(3), 1–9.
Lawson, D. A., et al. (2015). Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature, 526(7571), 131–135.
Luzzi, K. J., et al. (1998). Dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Multistep Nature of Metastatic Inefficiency, 153, 865–873.
Chen, D., et al. (2016). The neuropeptides FLP-2 and PDF-1 act in concert to arouse Caenorhabditis elegans locomotion. Genetics, 204(3), 1151–1159.
Barnes, D. G., et al. (2013). Embedding and publishing interactive, 3-dimensional, scientific figures in portable document format (PDF) files. PLoS One, 8(9), 1559–1564.
Lenos, K. J., et al. (2018). Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer. Nature Cell Biology, 20(10), 1193–1202.
Li, F., et al. (2019). Retinoblastoma inactivation induces a Protumoral microenvironment via enhanced CCL2 secretion. Cancer Research, 79(15), 3903–3915.
Wilson, J. L., et al. (2020). Inverse data-driven modeling and multiomics analysis reveals Phgdh as a metabolic checkpoint of macrophage polarization and proliferation. Cell Reports, 30(5), 1542–1552.
Plaks, V., et al. (2015). The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell, 16(3), 225–238.
Wortzel, I., et al. (2019). Exosome-mediated metastasis: Communication from a distance. Developmental Cell, 49(3), 347–360.
Devhare, P. B., & Ray, R. B. (2018). Extracellular vesicles: Novel mediator for cell to cell communications in liver pathogenesis. Molecular Aspects of Medicine, 60, 115–122.
Rauner, G., et al. (2018). High expression of CD200 and CD200R1 distinguishes stem and progenitor cell populations within mammary repopulating units. Stem Cell Reports, 11(1), 288–302.
Mathieu, M., et al. (2019). Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature Cell Biology, 21(1), 9–17.
Bonsergent, E., et al. (2021). Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nature Communications, 12(1), 1–11.
Ratajczak, M. Z., & Ratajczak, J. (2020). Extracellular microvesicles/exosomes: Discovery, disbelief, acceptance, and the future? Leukemia, 34(12), 3126–3135.
Pegtel, D. M., & Gould, S. J. (2019). Exosomes. Annual Review of Biochemistry, 88, 487–514.
Spilak, A., et al. (2021). Implications and pitfalls for cancer diagnostics exploiting extracellular vesicles. Advanced Drug Delivery Reviews, 175, 1–18.
Kalluri, R., & LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. science, 367, 1–15.
Galasso, M., et al. (2012). MicroRNA expression signatures in solid malignancies. The Cancer Journal, 18, 238–243.
Xie, Y., et al. (2019). The role of exosomal noncoding RNAs in cancer. Molecular Cancer, 18(1), 1–10.
Pomatto, M. A. C., et al. (2019). Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol Ther Methods Clin Dev, 13, 133–144.
Sun, Z., et al. (2018). Effect of exosomal miRNA on cancer biology and clinical applications. Molecular Cancer, 17(1), 1–19.
Zhang, L., & Yu, D. (2019). Exosomes in cancer development, metastasis, and immunity. Biochimica Et Biophysica Acta. Reviews on Cancer, 1871(2), 455–468.
Cavallari, C., et al. (2020). Extracellular vesicles in the tumour microenvironment: Eclectic supervisors. International Journal of Molecular Sciences, 21(18), 1–21.
Zeng, Z., et al. (2018). Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nature Communications, 9(1), 1–14.
Fang, T., et al. (2018). Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nature Communications, 9(1), 1–13.
Yu, L., et al. (2021). Exosomes derived from osteogenic tumor activate osteoclast differentiation and concurrently inhibit osteogenesis by transferring COL1A1-targeting miRNA-92a-1-5p. J Extracell Vesicles, 10(3), 1–27.
Zhao, S., et al. (2020). Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. Journal of Hematology & Oncology, 13(1), 1–19.
Lopatina, T., et al. (2020). Targeting IL-3Ralpha on tumor-derived endothelial cells blunts metastatic spread of triple-negative breast cancer via extracellular vesicle reprogramming. Oncogenesis, 9(10), 1–14.
Lopatina, T., et al. (2020). Extracellular vesicles released by tumor endothelial cells spread immunosuppressive and transforming signals through various recipient cells. Frontiers in Cell and Development Biology, 8, 1–14.
Lombardo, G., et al. (2018). IL-3R-alpha blockade inhibits tumor endothelial cell-derived extracellular vesicle (EV)-mediated vessel formation by targeting the beta-catenin pathway. Oncogene, 37(9), 1175–1191.
Yang, B., et al. (2020). High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene, 39(42), 6529–6543.
Wang, L., et al. (2019). CD103-positive CSC exosome promotes EMT of clear cell renal cell carcinoma: Role of remote MiR-19b-3p. Molecular Cancer, 18(1), 1–15.
Wang, Z. F., et al. (2019). Glioma stem cells-derived exosomal miR-26a promotes angiogenesis of microvessel endothelial cells in glioma. Journal of Experimental & Clinical Cancer Research, 38(1), 1–15.
Lopez de Andres, J., et al. (2020). Cancer stem cell secretome in the tumor microenvironment: A key point for an effective personalized cancer treatment. Journal of Hematology & Oncology, 13(1), 1–22.
Somasundaram, R., & Herlyn, M. (2012). Melanoma exosomes: messengers of metastasis. Nature Medicine, 18(6), 853–854.
Kim, J., et al. (2018). Replication study: Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Elife, 7, 1–17.
Vignard, V., et al. (2020). MicroRNAs in tumor exosomes drive immune escape in melanoma. Cancer Immunology Research, 8(2), 255–267.
Wang, J., et al. (2017). Comparison of tumor biology of two distinct cell sub-populations in lung cancer stem cells. Oncotarget, 8(57), 96852–96864.
Sun, Z., et al. (2018). Sec23a mediates miR-200c augmented oligometastatic to polymetastatic progression. EBioMedicine, 37, 47–55.
Song, L., et al. (2019). KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nature Communications, 10(1), 1–13.
Makarova, J., et al. (2021). Extracellular miRNAs and cell-cell communication: Problems and prospects. Trends in Biochemical Sciences, 46(8), 640–651.
Wu, K., et al. (2021). Exosomal miR-19a and IBSP cooperate to induce osteolytic bone metastasis of estrogen receptor-positive breast cancer. Nature Communications, 12(1), 1–18.
Fang, J. H., et al. (2018). Hepatoma cell-secreted exosomal microRNA-103 increases vascular permeability and promotes metastasis by targeting junction proteins. Hepatology, 68(4), 1459–1475.
Liu, C., et al. (2017). MicroRNA-141 suppresses prostate cancer stem cells and metastasis by targeting a cohort of pro-metastasis genes. Nature Communications, 8, 1–14.
Barkan, D., et al. (2008). Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Research, 68(15), 6241–6250.
Sun, Z., et al. (2020). S100A8 transported by SEC23A inhibits metastatic colonization via autocrine activation of autophagy. Cell Death & Disease, 11(8), 1–13.
Ono, M., et al. (2014). Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Science Signaling, 7(332), 1–10.
Temoche-Diaz, M. M., et al. (2019). Distinct mechanisms of microRNA sorting into cancer cell-derived extracellular vesicle subtypes. Elife, 8, 1–34.
Grzywa, T. M., et al. (2017). Intratumor and Intertumor heterogeneity in melanoma. Translational Oncology, 10(6), 956–975.
Zhang, X., et al. (2015). Exosomes in cancer: Small particle, big player. Journal of Hematology & Oncology, 8, 1–13.
Wang, L., et al. (2020). Lung CSC-derived exosomal miR-210-3p contributes to a pro-metastatic phenotype in lung cancer by targeting FGFRL1. Journal of Cellular and Molecular Medicine, 24(11), 6324–6339.
Li, J., et al. (2021). Hypoxic glioma stem cell-derived exosomes containing Linc01060 promote progression of glioma by regulating the MZF1/c-Myc/HIF1alpha Axis. Cancer Research, 81(1), 114–128.
Hardin, H., et al. (2018). Thyroid cancer stem-like cell exosomes: Regulation of EMT via transfer of lncRNAs. Laboratory Investigation, 98(9), 1133–1142.
Yang, Z., et al. (2020). Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cellular Oncology (Dordrecht), 43(1), 123–136.
Hwang, W. L., et al. (2019). Tumor stem-like cell-derived exosomal RNAs prime neutrophils for facilitating tumorigenesis of colon cancer. Journal of Hematology & Oncology, 12(1), 1–17.
Naseri, M., et al. (2021). Dendritic cells loaded with exosomes derived from cancer stem cell-enriched spheroids as a potential immunotherapeutic option. Journal of Cellular and Molecular Medicine, 25(7), 3312–3326.
Yuan, Y., et al. (2021). Exosomal O-GlcNAc transferase from esophageal carcinoma stem cell promotes cancer immunosuppression through up-regulation of PD-1 in CD8(+) T cells. Cancer Letters, 500, 98–106.
Sun, Z., et al. (2020). Glioblastoma stem cell-derived exosomes enhance Stemness and Tumorigenicity of glioma cells by transferring Notch1 protein. Cellular and Molecular Neurobiology, 40(5), 767–784.
Dai, W., et al. (2020). Exosomal lncRNA DOCK9-AS2 derived from cancer stem cell-like cells activated Wnt/beta-catenin pathway to aggravate stemness, proliferation, migration, and invasion in papillary thyroid carcinoma. Cell Death & Disease, 11(9), 1–17.
Cha, S. Y., et al. (2017). Clinical impact of microRNAs associated with Cancer stem cells as a prognostic factor in ovarian carcinoma. Journal of Cancer, 8(17), 3538–3547.
Conigliaro, A., et al. (2015). CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Molecular Cancer, 14, 1–11.
Yoshikawa, K., et al. (2021). Diagnostic predictability of miR-4535 and miR-1915-5p expression in amniotic fluid for foetal morbidity of infection. Placenta, 114, 68–75.
Hannafon, B. N., et al. (2016). Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Research, 18(1), 1–14.
Hydbring, P., et al. (2018). Exosomal RNA-profiling of pleural effusions identifies adenocarcinoma patients through elevated miR-200 and LCN2 expression. Lung Cancer, 124, 45–52.
Lee, C. H., et al. (2018). Discovery of a diagnostic biomarker for colon cancer through proteomic profiling of small extracellular vesicles. BMC Cancer, 18(1), 1–11.
Kenific, C. M., et al. (2010). Autophagy and metastasis: Another double-edged sword. Current Opinion in Cell Biology, 22(2), 241–245.
Lu, Z., et al. (2008). The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. The Journal of Clinical Investigation, 118(12), 3917–3929.
Mowers, E. E., et al. (2017). Autophagy in cancer metastasis. Oncogene, 36(12), 1619–1630.
Su, Z., et al. (2015). Apoptosis, autophagy, necroptosis, and cancer metastasis. Molecular Cancer, 14, 1–14.
Acknowledgments
I would like to thank all the students in the lab for their help and the members of the research group for their efforts. I would also like to thank my advisors for their guidance. In addition, thanks for the funding supports of the National Natural Science Fund (Grant No. 82073277 and 82173247), the Science and Technology Project Affiliated to the Education Department of Chongqing (Grant No. KJQN202100404), Natural Science Fund of Chongqing (Grant No. cstc2019jcyj-msxmX0868) and Science and Technology Project of Chongqing Yuzhong District (Grant No. 20200110).
Funding
This work was supported by the National Natural Science Fund (Grant No. 82073277 and 82173247), the Science and Technology Project Affiliated to the Education Department of Chongqing (Grant No. KJQN202100404), Natural Science Fund of Chongqing (Grant No. cstc2019jcyj-msxmX0868) and Science and Technology Project of Chongqing Yuzhong District (Grant No. 20200110).
Author information
Authors and Affiliations
Contributions
Doudou Liu and Xiaoshuang Li completed the molecular experiment, Bin Zeng and Qiting Zhao completed the animal experiment, Hao Chen was responsible for exosome extraction, Yuhan Zhang and Yuting Chen completed the analysis of sequencing data. Doudou Liu wrote the article, Jianyu Wang and H. Rosie Xing were responsible for the overall design of this study and the revision and improvement of the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
All animal work was conducted in accordance with an approved protocol and carried out in accordance with the institutional animal welfare guidelines of the Chongqing Medical University. The license number of experimental animal is SYXK2018–0003.
Consent to Participate
All authors agreed to participate in the study of the subject.
Consent for Publication
All authors agree to revise the paper for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Supplementary Figure 1
A. Efficiency of transfection of miR-4535 mimics into MSC exosomes by electroporation, **refers to p < 0.01. B. Expression of miR-4535 in MPC cells was increased after MPC cells co-cultured with MSC exosomes that overexpressed miR-4535, *refers to p < 0.05. C-D. Overexpression of miR-4535 in MSC exosomes promoted the migration (C) and invasion (D) of MPC cells, the bar is 210 μm, **refers to p < 0.01. E. The level of miR-4535 in MSC exosomes was increased when miR-4535 was overexpressed in MSC cells, ***refers to p < 0.001. F. After overexpression of miR-4535 in MSC cells, MSC exosomes were co-cultured with MPC, and the expression of miR-4535 in MPC was also increased, *refers to p < 0.05. G-H. After miR-4535 overexpression in MSC cells, MSC exosomes promoted the migration (G) and invasion (H) of MPC cells, the bar is 210 μm, ***refers to p < 0.001. I. After inhibiting the expression of miR-4535 in MSC cells with the inhibitor, the level of miR-4535 in exosomes was also decreased, ***refers to p < 0.001. (PNG 2269 kb)

Supplementary Figure 2
A. Predicted binding site and score of miRNA-4535 and ATG13 in TargetScan. B. Predicted binding sites and mutation sites of miRNA-4535 and ATG13. C. Relative luciferase activities were analyzed in 293 T cells co-transfected with ATG13 3 ′ -UTR (wild type or mutant) reporter plasmid, and microRNA 4535 mimic or NC mimics. (PNG 475 kb)

Supplementary Figure 3
A. Assessment of the stemness of MSC cells by the spheroid formation ability of 1000 cells in a 6-well plate. The scale bar is 300 μm. B. Assessment of the stemness of MSC cells by single cell cloning rate in 96-well plates after the expression of miRNA 4535 was inhibited in MSC cells. C. The expression of stemness genes after the expression of miRNA 4535 was inhibited in MSC cells. ***refers to P < 0.001 and **refers to p < 0.01. (PNG 1728 kb)
Rights and permissions
About this article
Cite this article
Liu, D., Li, X., Zeng, B. et al. Exosomal microRNA-4535 of Melanoma Stem Cells Promotes Metastasis by Inhibiting Autophagy Pathway. Stem Cell Rev and Rep 19, 155–169 (2023). https://doi.org/10.1007/s12015-022-10358-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10358-4