Abstract
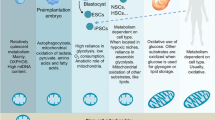
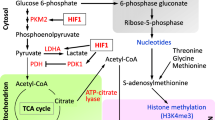
Pluripotent stem cells (PSCs), including embryonic stem cells and induced pluripotent stem cells (iPSCs), can be applicable for regenerative medicine. They strangely rely on glycolysis metabolism akin to aerobic glycolysis in cancer cells. Upon differentiation, PSCs undergo a metabolic shift from glycolysis to oxidative phosphorylation (OXPHOS). The metabolic shift depends on organelles maturation, transcriptome modification, and metabolic switching. Besides, metabolism-driven chromatin regulation is necessary for cell survival, self-renewal, proliferation, senescence, and differentiation. In this respect, mitochondria may serve as key organelle to adapt environmental changes with metabolic intermediates which are necessary for maintaining PSCs identity. The endoplasmic reticulum (ER) is another organelle whose role in cellular identity remains under-explored. The purpose of our article is to highlight the recent progress on these two organelles’ role in maintaining PSCs redox status focusing on metabolism. Topics include redox status, metabolism regulation, mitochondrial dynamics, and ER stress in PSCs. They relate to the maintenance of stem cell properties and subsequent differentiation of stem cells into specific cell types.
Graphical abstract






Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- Ac:
-
acetylation marks on histones
- Ac-CoA:
-
acetyl-CoA
- ACLY:
-
ATP-citrate lyase
- ATF4:
-
Activation of transcription factor 4
- ATF6:
-
Activating transcription factor 6
- CHOP:
-
CCAAT/ enhancer-binding protein homologous-protein
- CTBP:
-
c-terminal binding proteins
- DRP1:
-
Dynamin-related protein 1
- ERAD:
-
ER-associated degradation
- eIF2α:
-
eukaryotic initiating factor 2α
- FOXO1:
-
Forkhead box O1
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GDAP1:
-
Ganglioside-induced differentiation associated protein1
- GRP75:
-
Glucose-regulated protein 75
- GRP78:
-
Glucose regulated protein 78
- GSK3β:
-
Glycogen synthase kinase3β
- GPX:
-
glutathione peroxidase
- HIF1-α:
-
Hypoxia-inducible factor1-α
- HRE:
-
Hypoxia-responsive elements
- HAT:
-
Histone acetyl transferase
- hESCs:
-
human embryonic stem cells
- IDO1:
-
Indoleamine 2,3-dioxygenase 1
- IMM:
-
Inner membrane
- IP3R:
-
Inositol 1,4,5-trisphosphate receptor
- c-Jun N-terminal kinase:
-
JNK
- JHDM:
-
JmjC-domain containing histone demethylases
- MFF:
-
Mitochondrial fission factor
- mtDNA:
-
Mitochondrial DNA
- mTOR:
-
mammalian target of rapamycin
- Met:
-
Methylation
- NAD:
-
Nicotinamide adenine dinucleotide
- Nrf2:
-
Nuclear factor erythroid 2 related factor 2
- OMM:
-
Outer membrane
- OPA1:
-
Optic atrophy protein 1
- PSCs:
-
Pluripotent stem cells
- PDK:
-
Pyruvate dehydrogenase kinase
- PDH:
-
Pyruvate dehydrogenase
- PINK1:
-
PGC1α, Peroxisome proliferator-activated receptor gamma coactivator1α
- PTEN:
-
induced putative kinase 1
- PERK:
-
Protein kinase-like endoplasmic reticulum kinase
- REX1:
-
Reduced expression 1
- ROS:
-
Reactive Oxygen Species
- SOD:
-
Superoxide dismutase
- SIRT:
-
Sirtuin
- TET:
-
Ten-eleven translocation
- UPR:
-
Unfolded protein response
- VDAC:
-
Voltage dependent anion channel
- XBP1:
-
X-box binding protein1
References
Mora, C., Serzanti, M., Consiglio, A., Memo, M., & Dell’Era, P. (2017). Clinical potentials of human pluripotent stem cells. Cell Biology and Toxicology. https://doi.org/10.1007/s10565-017-9384-y
Meamar, R., Nasr-Esfahani, M. H., Mousavi, S. A., & Basiri, K. (2013). Stem cell therapy in amyotrophic lateral sclerosis. Journal of Clinical Neuroscience. https://doi.org/10.1016/j.jocn.2013.04.024
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S., & Jones, J. M. (1998). Embryonic stem cell lines derived from human blastocysts. Science. https://doi.org/10.1126/science.282.5391.1145
Boroviak, T., Loos, R., Lombard, P., Okahara, J., Behr, R., Sasaki, E., Nichols, J., Smith, A., & Bertone, P. (2015). Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis. Developmental Cell. https://doi.org/10.1016/j.devcel.2015.10.011
Ha, T. W., Jeong, J. H., Shin, H., Kim, H. K., Im, J. S., Song, B. H., Hanna, J., Oh, J. S., Woo, D. H., Han, J., & Lee, M. R. (2020). Characterization of endoplasmic reticulum (ER) in human pluripotent stem cells revealed increased susceptibility to cell death upon ER stress. Cells. https://doi.org/10.3390/cells9051078
Oh, S. K., Kim, H. S., Ahn, H. J., Seol, H. W., Kim, Y. Y., Park, Y. B., Yoon, C. J., Kim, D. W., Kim, S. H., & Moon, S. Y. (2005). Derivation and characterization of new human embryonic stem cell lines: SNUhES1, SNUhES2, and SNUhES3. Stem Cells. https://doi.org/10.1634/stemcells.2004-0122
Barreto, A., Gonzalez, J. M., Kabingu, E., Asea, A., & Fiorentino, S. (2003). Stress-induced release of HSC70 from human tumors. Cellular Immunology. https://doi.org/10.1016/s0008-8749(03)00115-1
Buszczak, M., Signer, R. A., & Morrison, S. J. (2014). Cellular differences in protein synthesis regulate tissue homeostasis. Cell. https://doi.org/10.1016/j.cell.2014.09.016
Vilchez, D., Boyer, L., Morantte, I., Lutz, M., Merkwirth, C., Joyce, D., Spencer, B., Page, L., Masliah, E., Berggren, W. T., Gage, F. H., & Dillin, A. (2012). Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature. https://doi.org/10.1038/nature11468
Yang, Y., Cheung, H.H., Tu, J., Miu, K.K., &Chan, W.Y. (2016). New insights into the unfolded protein response in stem cells. Oncotarget,
Lin, J. H., Li, H., Yasumura, D., Cohen, H. R., Zhang, C., Panning, B., Shokat, K. M., Lavail, M. M., & Walter, P. (2007). IRE1 signaling affects cell fate during the unfolded protein response. Science. https://doi.org/10.1126/science.1146361
Hosseini, M., Shaygannia, E., Rahmani, M., Eskandari, A., Golsefid, A. A., Tavalaee, M., Gharagozloo, P., Drevet, J. R., & Nasr-Esfahani, M. H. (2020). Endoplasmic reticulum stress (ER stress) and unfolded protein response (UPR) occur in a rat varicocele testis model. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2020/5909306
Bantug, G. R., Fischer, M., Grahlert, J., Balmer, M. L., Unterstab, G., Develioglu, L., Steiner, R., Zhang, L., Costa, A. S. H., Gubser, P. M., Burgener, A. V., Sauder, U., Loliger, J., Belle, R., Dimeloe, S., Lotscher, J., Jauch, A., Recher, M., Honger, G., et al. (2018). Mitochondria-endoplasmic reticulum contact sites function as Immunometabolic hubs that orchestrate the rapid recall response of memory CD8(+) T cells. Immunity. https://doi.org/10.1016/j.immuni.2018.02.012
Sart, S., Song, L., & Li, Y. (2015). Controlling redox status for stem cell survival, expansion, and differentiation. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2015/105135
Perales-Clemente, E., Folmes, C. D., & Terzic, A. (2014). Metabolic regulation of redox status in stem cells. Antioxidants & Redox Signaling. https://doi.org/10.1089/ars.2014.6000
Wang, Q. Y., Liu, Z. S., Wang, J., Wang, H. X., Li, A., Yang, Y., Wang, X. Z., Zhao, Y. Q., Han, Q. Y., Cai, H., Liang, B., Song, N., Li, W. H., & Li, T. (2014). Glutathione peroxidase-1 is required for self-renewal of murine embryonic stem cells. Biochemical and Biophysical Research Communications. https://doi.org/10.1016/j.bbrc.2014.04.139
Esteban, M. A., Wang, T., Qin, B., Yang, J., Qin, D., Cai, J., Li, W., Weng, Z., Chen, J., Ni, S., Chen, K., Li, Y., Liu, X., Xu, J., Zhang, S., Li, F., He, W., Labuda, K., Song, Y., et al. (2010). Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. https://doi.org/10.1016/j.stem.2009.12.001
Liu, J. C., Guan, X., Ryan, J. A., Rivera, A. G., Mock, C., Agrawal, V., Letai, A., Lerou, P. H., & Lahav, G. (2013). High mitochondrial priming sensitizes hESCs to DNA-damage-induced apoptosis. Cell Stem Cell. https://doi.org/10.1016/j.stem.2013.07.018
Kim, S. U., Park, Y. H., Kim, J. M., Sun, H. N., Song, I. S., Huang, S. M., Lee, S. H., Chae, J. I., Hong, S., Sik Choi, S., Choi, S. C., Lee, T. H., Kang, S. W., Rhee, S. G., Chang, K. T., Lee, S. H., Yu, D. Y., & Lee, D. S. (2014). Dominant role of peroxiredoxin/JNK axis in stemness regulation during neurogenesis from embryonic stem cells. Stem Cells. https://doi.org/10.1002/stem.1593
Zhou, L., Zheng, B., Tang, L., Huang, Y., & Zhu, D. (2014). Involvement of PIKE in icariin induced cardiomyocyte differentiation from murine embryonic stem cells. Pharmazie.
Nazem, S., Rabiee, F., Ghaedi, K., Babashah, S., Sadeghizadeh, M., & Nasr-Esfahani, M.H. (2018). Fndc5 knockdown induced suppression of mitochondrial integrity and significantly decreased cardiac differentiation of mouse embryonic stem cells. Journal of Cellular Biochemistry. https://doi.org/10.1002/jcb.26590
Kopacz, A., Kloska, D., Forman, H. J., Jozkowicz, A., & Grochot-Przeczek, A. (2020). Beyond repression of Nrf2: An update on Keap1. Free Radical Biology & Medicine. https://doi.org/10.1016/j.freeradbiomed.2020.03.023
Ishii, T., & andMann, G.E. (2014). Redox status in mammalian cells and stem cells during culture in vitro: Critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biology. https://doi.org/10.1016/j.redox.2014.04.008
Jang, J., Wang, Y., Kim, H. S., Lalli, M. A., & Kosik, K. S. (2014). Nrf2, a regulator of the proteasome, controls self-renewal and pluripotency in human embryonic stem cells. Stem Cells. https://doi.org/10.1002/stem.1764
Hu, Q., Khanna, P., Ee Wong, B.S., Lin Heng, Z.S., Subhramanyam, C.S., Thanga, L.Z., Sing Tan, S.W., &Baeg, G.H. (2018). Oxidative stress promotes exit from the stem cell state and spontaneous neuronal differentiation. Oncotarget, https://doi.org/10.18632/oncotarget.23786.
Varum, S., Momcilovic, O., Castro, C., Ben-Yehudah, A., Ramalho-Santos, J., & Navara, C. S. (2009). Enhancement of human embryonic stem cell pluripotency through inhibition of the mitochondrial respiratory chain. Stem Cell Research. https://doi.org/10.1016/j.scr.2009.07.002
Facucho-Oliveira, J. M., Alderson, J., Spikings, E. C., Egginton, S., & St John, J. C. (2007). Mitochondrial DNA replication during differentiation of murine embryonic stem cells. Journal of Cell Science. https://doi.org/10.1242/jcs.016972
Sperber, H., Mathieu, J., Wang, Y., Ferreccio, A., Hesson, J., Xu, Z., Fischer, K. A., Devi, A., Detraux, D., Gu, H., Battle, S. L., Showalter, M., Valensisi, C., Bielas, J. H., Ericson, N. G., Margaretha, L., Robitaille, A. M., Margineantu, D., Fiehn, O., et al. (2015). The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nature Cell Biology. https://doi.org/10.1038/ncb3264
Gu, W., Gaeta, X., Sahakyan, A., Chan, A. B., Hong, C. S., Kim, R., Braas, D., Plath, K., Lowry, W. E., & Christofk, H. R. (2016). Glycolytic metabolism plays a functional role in regulating human pluripotent stem cell state. Cell Stem Cell. https://doi.org/10.1016/j.stem.2016.08.008
Prigione, A., Fauler, B., Lurz, R., Lehrach, H., &Adjaye, J. (2010). The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem cells,
Zhang, J., Zhao, J., Dahan, P., Lu, V., Zhang, C., Li, H., & Teitell, M. A. (2018). Metabolism in pluripotent stem cells and early mammalian development. Cell Metabolism. https://doi.org/10.1016/j.cmet.2018.01.008
Zhu, S., Li, W., Zhou, H., Wei, W., Ambasudhan, R., Lin, T., Kim, J., Zhang, K., & Ding, S. (2010). Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. https://doi.org/10.1016/j.stem.2010.11.015
Folmes, C. D., Nelson, T. J., Martinez-Fernandez, A., Arrell, D. K., Lindor, J. Z., Dzeja, P. P., Ikeda, Y., Perez-Terzic, C., & Terzic, A. (2011). Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metabolism. https://doi.org/10.1016/j.cmet.2011.06.011
Mlody, B., & Prigione, A. (2016). A glycolytic solution for pluripotent stem cells. Cell Stem Cell. https://doi.org/10.1016/j.stem.2016.09.005
Shyh-Chang, N., & andDaley, G.Q. (2015). Metabolic switches linked to pluripotency and embryonic stem cell differentiation. Cell Metabolism. https://doi.org/10.1016/j.cmet.2015.02.011
Lisowski, P., Kannan, P., Mlody, B., &Prigione, A. (2018). Mitochondria and the dynamic control of stem cell homeostasis. EMBO rep, https://doi.org/10.15252/embr.201745432.
Lu, C., & Thompson, C. B. (2012). Metabolic regulation of epigenetics. Cell Metabolism. https://doi.org/10.1016/j.cmet.2012.06.001
Moussaieff, A., Rouleau, M., Kitsberg, D., Cohen, M., Levy, G., Barasch, D., Nemirovski, A., Shen-Orr, S., Laevsky, I., Amit, M., Bomze, D., Elena-Herrmann, B., Scherf, T., Nissim-Rafinia, M., Kempa, S., Itskovitz-Eldor, J., Meshorer, E., Aberdam, D., & Nahmias, Y. (2015). Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metabolism. https://doi.org/10.1016/j.cmet.2015.02.002
Warburg, O. (1956). On respiratory impairment in cancer cells. Science.
Shyh-Chang, N., & Ng, H. H. (2017). The metabolic programming of stem cells. Genes & Development. https://doi.org/10.1101/gad.293167.116
Ward, P. S., & andThompson, C.B. (2012). Metabolic reprogramming: A cancer hallmark even Warburg did not anticipate. Cancer Cell. https://doi.org/10.1016/j.ccr.2012.02.014
Li, X. L., Eishi, Y., Bai, Y. Q., Sakai, H., Akiyama, Y., Tani, M., Takizawa, T., Koike, M., & Yuasa, Y. (2004). Expression of the SRY-related HMG box protein SOX2 in human gastric carcinoma. International Journal of Oncology.
Ling, G. Q., Chen, D. B., Wang, B. Q., & Zhang, L. S. (2012). Expression of the pluripotency markers Oct3/4, Nanog and Sox2 in human breast cancer cell lines. Oncology Letters. https://doi.org/10.3892/ol.2012.916
Ben-Porath, I., Thomson, M. W., Carey, V. J., Ge, R., Bell, G. W., Regev, A., & Weinberg, R. A. (2008). An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nature Genetics. https://doi.org/10.1038/ng.127
Shakya, A., Cooksey, R., Cox, J. E., Wang, V., McClain, D. A., & Tantin, D. (2009). Oct1 loss of function induces a coordinate metabolic shift that opposes tumorigenicity. Nature Cell Biology. https://doi.org/10.1038/ncb1840
Lees, J. G., Gardner, D. K., & Harvey, A. J. (2020). Nicotinamide adenine dinucleotide induces a bivalent metabolism and maintains pluripotency in human embryonic stem cells. Stem Cells. https://doi.org/10.1002/stem.3152
Safaeinejad, Z., Nabiuni, M., Peymani, M., Ghaedi, K., Nasr-Esfahani, M. H., & Baharvand, H. (2017). Resveratrol promotes human embryonic stem cells self-renewal by targeting SIRT1-ERK signaling pathway. European Journal of Cell Biology. https://doi.org/10.1016/j.ejcb.2017.08.002
Zhang, Z. N., Chung, S. K., Xu, Z., & Xu, Y. (2014). Oct4 maintains the pluripotency of human embryonic stem cells by inactivating p53 through Sirt1-mediated deacetylation. Stem Cells. https://doi.org/10.1002/stem.1532
Zhao, T., & andXu, Y. (2010). p53 and stem cells: New developments and new concerns. Trends in Cell Biology. https://doi.org/10.1016/j.tcb.2009.12.004
Ito, S., Shen, L., Dai, Q., Wu, S. C., Collins, L. B., Swenberg, J. A., He, C., & Zhang, Y. (2011). Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. https://doi.org/10.1126/science.1210597
Wang, T., Chen, K., Zeng, X., Yang, J., Wu, Y., Shi, X., Qin, B., Zeng, L., Esteban, M. A., Pan, G., & Pei, D. (2011). The histone demethylases Jhdm1a/1b enhance somatic cell reprogramming in a vitamin-C-dependent manner. Cell Stem Cell. https://doi.org/10.1016/j.stem.2011.10.005
TeSlaa, T., Chaikovsky, A. C., Lipchina, I., Escobar, S. L., Hochedlinger, K., Huang, J., Graeber, T. G., Braas, D., & Teitell, M. A. (2016). Alpha-ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metabolism. https://doi.org/10.1016/j.cmet.2016.07.002
Carey, B. W., Finley, L. W., Cross, J. R., Allis, C. D., & Thompson, C. B. (2015). Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. https://doi.org/10.1038/nature13981
Viswanathan, S. R., & andDaley, G.Q. (2010). Lin28: A microRNA regulator with a macro role. Cell. https://doi.org/10.1016/j.cell.2010.02.007
Peng, S., Chen, L. L., Lei, X. X., Yang, L., Lin, H., Carmichael, G. G., & Huang, Y. (2011). Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells. https://doi.org/10.1002/stem.591
Narva, E., Rahkonen, N., Emani, M. R., Lund, R., Pursiheimo, J. P., Nasti, J., Autio, R., Rasool, O., Denessiouk, K., Lahdesmaki, H., Rao, A., & Lahesmaa, R. (2012). RNA-binding protein L1TD1 interacts with LIN28 via RNA and is required for human embryonic stem cell self-renewal and cancer cell proliferation. Stem Cells. https://doi.org/10.1002/stem.1013
Zhang, J., Ratanasirintrawoot, S., Chandrasekaran, S., Wu, Z., Ficarro, S. B., Yu, C., Ross, C. A., Cacchiarelli, D., Xia, Q., Seligson, M., Shinoda, G., Xie, W., Cahan, P., Wang, L., Ng, S. C., Tintara, S., Trapnell, C., Onder, T., Loh, Y. H., et al. (2016). LIN28 regulates stem cell metabolism and conversion to primed pluripotency. Cell Stem Cell. https://doi.org/10.1016/j.stem.2016.05.009
Takahashi, K., & andYamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. https://doi.org/10.1016/j.cell.2006.07.024
Hanna, J., Saha, K., Pando, B., van Zon, J., Lengner, C. J., Creyghton, M. P., van Oudenaarden, A., & Jaenisch, R. (2009). Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. https://doi.org/10.1038/nature08592
Buganim, Y., Faddah, D. A., Cheng, A. W., Itskovich, E., Markoulaki, S., Ganz, K., Klemm, S. L., van Oudenaarden, A., & Jaenisch, R. (2012). Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell. https://doi.org/10.1016/j.cell.2012.08.023
Blackwell, T. K., Huang, J., Ma, A., Kretzner, L., Alt, F. W., Eisenman, R. N., & Weintraub, H. (1993). Binding of myc proteins to canonical and noncanonical DNA sequences. Molecular and Cellular Biology. https://doi.org/10.1128/mcb.13.9.5216
Cliff, T. S., Wu, T., Boward, B. R., Yin, A., Yin, H., Glushka, J. N., Prestegaard, J. H., & Dalton, S. (2017). MYC controls human pluripotent stem cell fate decisions through regulation of metabolic flux. Cell Stem Cell. https://doi.org/10.1016/j.stem.2017.08.018
Varlakhanova, N. V., Cotterman, R. F., deVries, W. N., Morgan, J., Donahue, L. R., Murray, S., Knowles, B. B., & Knoepfler, P. S. (2010). Myc maintains embryonic stem cell pluripotency and self-renewal. Differentiation. https://doi.org/10.1016/j.diff.2010.05.001
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. https://doi.org/10.1016/j.cell.2007.11.019
Folmes, C. D., Martinez-Fernandez, A., Faustino, R. S., Yamada, S., Perez-Terzic, C., Nelson, T. J., & Terzic, A. (2013). Nuclear reprogramming with c-Myc potentiates glycolytic capacity of derived induced pluripotent stem cells. Journal of Cardiovascular Translational Research. https://doi.org/10.1007/s12265-012-9431-2
Zhang, H., Badur, M. G., Divakaruni, A. S., Parker, S. J., Jager, C., Hiller, K., Murphy, A. N., & Metallo, C. M. (2016). Distinct metabolic states can support self-renewal and lipogenesis in human pluripotent stem cells under different culture conditions. Cell Reports. https://doi.org/10.1016/j.celrep.2016.06.102
Ogg, S., Paradis, S., Gottlieb, S., Patterson, G.I., Lee, L., Tissenbaum, H.A., &Ruvkun, G. (1997). The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature, https://doi.org/10.1038/40194.
Essers, M. A., Weijzen, S., de Vries-Smits, A. M., Saarloos, I., de Ruiter, N. D., Bos, J. L., & Burgering, B. M. (2004). FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. The EMBO Journal. https://doi.org/10.1038/sj.emboj.7600476
Zhang, X., Rielland, M., Yalcin, S., & Ghaffari, S. (2011). Regulation and function of FoxO transcription factors in normal and cancer stem cells: What have we learned? Current Drug Targets. https://doi.org/10.2174/138945011796150325
Yalcin, S., Zhang, X., Luciano, J. P., Mungamuri, S. K., Marinkovic, D., Vercherat, C., Sarkar, A., Grisotto, M., Taneja, R., & Ghaffari, S. (2008). Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. The Journal of Biological Chemistry. https://doi.org/10.1074/jbc.M800517200
Zhang, X., Yalcin, S., Lee, D. F., Yeh, T. Y., Lee, S. M., Su, J., Mungamuri, S. K., Rimmele, P., Kennedy, M., Sellers, R., Landthaler, M., Tuschl, T., Chi, N. W., Lemischka, I., Keller, G., & Ghaffari, S. (2011). FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nature Cell Biology. https://doi.org/10.1038/ncb2293
Miyamoto, K., Araki, K. Y., Naka, K., Arai, F., Takubo, K., Yamazaki, S., Matsuoka, S., Miyamoto, T., Ito, K., Ohmura, M., Chen, C., Hosokawa, K., Nakauchi, H., Nakayama, K., Nakayama, K. I., Harada, M., Motoyama, N., Suda, T., & Hirao, A. (2007). Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. https://doi.org/10.1016/j.stem.2007.02.001
Renault, V. M., Rafalski, V. A., Morgan, A. A., Salih, D. A., Brett, J. O., Webb, A. E., Villeda, S. A., Thekkat, P. U., Guillerey, C., Denko, N. C., Palmer, T. D., Butte, A. J., & Brunet, A. (2009). FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. https://doi.org/10.1016/j.stem.2009.09.014
Yu, Y., Liang, D., Tian, Q., Chen, X., Jiang, B., Chou, B. K., Hu, P., Cheng, L., Gao, P., Li, J., & Wang, G. (2014). Stimulation of somatic cell reprogramming by ERas-Akt-FoxO1 signaling axis. Stem Cells. https://doi.org/10.1002/stem.1447
Yamagata, K., Daitoku, H., Takahashi, Y., Namiki, K., Hisatake, K., Kako, K., Mukai, H., Kasuya, Y., & Fukamizu, A. (2008). Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Molecular Cell. https://doi.org/10.1016/j.molcel.2008.09.013
Wang, K., Zhang, T., Dong, Q., Nice, E. C., Huang, C., & Wei, Y. (2013). Redox homeostasis: The linchpin in stem cell self-renewal and differentiation. Cell Death & Disease. https://doi.org/10.1038/cddis.2013.50
Zhang, C. C., & Sadek, H. A. (2014). Hypoxia and metabolic properties of hematopoietic stem cells. Antioxidants & Redox Signaling. https://doi.org/10.1089/ars.2012.5019
Lee, H.J., Jung, Y.H., Choi, G.E., Kim, J.S., Chae, C.W., &Han, H.J. (2019). Role of HIF1alpha regulatory factors in stem cells. Int J stem cells, https://doi.org/10.15283/ijsc18109.
Mathieu, J., Zhou, W., Xing, Y., Sperber, H., Ferreccio, A., Agoston, Z., Kuppusamy, K. T., Moon, R. T., & Ruohola-Baker, H. (2014). Hypoxia-inducible factors have distinct and stage-specific roles during reprogramming of human cells to pluripotency. Cell Stem Cell. https://doi.org/10.1016/j.stem.2014.02.012
Arthur, S. A., Blaydes, J. P., & Houghton, F. D. (2019). Glycolysis regulates human embryonic stem cell self-renewal under hypoxia through HIF-2alpha and the glycolytic sensors CTBPs. Stem Cell Reports. https://doi.org/10.1016/j.stemcr.2019.02.005
Prigione, A., Rohwer, N., Hoffmann, S., Mlody, B., Drews, K., Bukowiecki, R., Blumlein, K., Wanker, E. E., Ralser, M., Cramer, T., & Adjaye, J. (2014). HIF1alpha modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2. Stem Cells. https://doi.org/10.1002/stem.1552
Jeong, C. H., Lee, H. J., Cha, J. H., Kim, J. H., Kim, K. R., Kim, J. H., Yoon, D. K., & Kim, K. W. (2007). Hypoxia-inducible factor-1 alpha inhibits self-renewal of mouse embryonic stem cells in vitro via negative regulation of the leukemia inhibitory factor-STAT3 pathway. The Journal of Biological Chemistry. https://doi.org/10.1074/jbc.M700534200
Piruat, J. I., & Lopez-Barneo, J. (2005). Oxygen tension regulates mitochondrial DNA-encoded complex I gene expression. The Journal of Biological Chemistry. https://doi.org/10.1074/jbc.M507044200
Kaelin Jr., W. G., & Lopez-Barneo, J. (2008). Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Molecular Cell. https://doi.org/10.1016/j.molcel.2008.04.009
Ward, P. S., & andThompson, C.B. (2012). Signaling in control of cell growth and metabolism. Cold Spring Harbor Perspectives in Biology. https://doi.org/10.1101/cshperspect.a006783
Lee, J. V., Carrer, A., Shah, S., Snyder, N. W., Wei, S., Venneti, S., Worth, A. J., Yuan, Z. F., Lim, H. W., Liu, S., Jackson, E., Aiello, N. M., Haas, N. B., Rebbeck, T. R., Judkins, A., Won, K. J., Chodosh, L. A., Garcia, B. A., Stanger, B. Z., et al. (2014). Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metabolism. https://doi.org/10.1016/j.cmet.2014.06.004
Watanabe, S., Umehara, H., Murayama, K., Okabe, M., Kimura, T., & Nakano, T. (2006). Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. https://doi.org/10.1038/sj.onc.1209307
Kennedy, S. G., Kandel, E. S., Cross, T. K., & Hay, N. (1999). Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Molecular and Cellular Biology. https://doi.org/10.1128/mcb.19.8.5800
Gottlob, K., Majewski, N., Kennedy, S., Kandel, E., Robey, R. B., & Hay, N. (2001). Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes & Development. https://doi.org/10.1101/gad.889901
Hossini, A. M., Quast, A. S., Plotz, M., Grauel, K., Exner, T., Kuchler, J., Stachelscheid, H., Eberle, J., Rabien, A., Makrantonaki, E., & Zouboulis, C. C. (2016). PI3K/AKT signaling pathway is essential for survival of induced pluripotent stem cells. PLoS One. https://doi.org/10.1371/journal.pone.0154770
Tang, Y., Jiang, Z., Luo, Y., Zhao, X., Wang, L., Norris, C., & Tian, X. C. (2014). Differential effects of Akt isoforms on somatic cell reprogramming. Journal of Cell Science. https://doi.org/10.1242/jcs.150029
Gangloff, Y. G., Mueller, M., Dann, S. G., Svoboda, P., Sticker, M., Spetz, J. F., Um, S. H., Brown, E. J., Cereghini, S., Thomas, G., & Kozma, S. C. (2004). Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Molecular and Cellular Biology. https://doi.org/10.1128/MCB.24.21.9508-9516.2004
Murakami, M., Ichisaka, T., Maeda, M., Oshiro, N., Hara, K., Edenhofer, F., Kiyama, H., Yonezawa, K., & Yamanaka, S. (2004). mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Molecular and Cellular Biology. https://doi.org/10.1128/MCB.24.15.6710-6718.2004
Lee, K. W., Yook, J. Y., Son, M. Y., Kim, M. J., Koo, D. B., Han, Y. M., & Cho, Y. S. (2010). Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway. Stem Cells and Development. https://doi.org/10.1089/scd.2009.0147
Zhou, J., Su, P., Wang, L., Chen, J., Zimmermann, M., Genbacev, O., Afonja, O., Horne, M. C., Tanaka, T., Duan, E., Fisher, S. J., Liao, J., Chen, J., & Wang, F. (2009). mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.0901854106
Ruiz, S., Panopoulos, A. D., Herrerias, A., Bissig, K. D., Lutz, M., Berggren, W. T., Verma, I. M., & Izpisua Belmonte, J. C. (2011). A high proliferation rate is required for cell reprogramming and maintenance of human embryonic stem cell identity. Current Biology. https://doi.org/10.1016/j.cub.2010.11.049
Chen, T., Shen, L., Yu, J., Wan, H., Guo, A., Chen, J., Long, Y., Zhao, J., & Pei, G. (2011). Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell. https://doi.org/10.1111/j.1474-9726.2011.00722.x
Wang, S., Xia, P., Ye, B., Huang, G., Liu, J., & Fan, Z. (2013). Transient activation of autophagy via Sox2-mediated suppression of mTOR is an important early step in reprogramming to pluripotency. Cell Stem Cell. https://doi.org/10.1016/j.stem.2013.10.005
Ball, H. J., Yuasa, H. J., Austin, C. J., Weiser, S., & Hunt, N. H. (2009). Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. The International Journal of Biochemistry & Cell Biology. https://doi.org/10.1016/j.biocel.2008.01.005
Khan, J. A., Forouhar, F., Tao, X., & Tong, L. (2007). Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opinion on Therapeutic Targets. https://doi.org/10.1517/14728222.11.5.695
Liu, X., Wang, M., Jiang, T., He, J., Fu, X., & &Xu, Y. (2019). IDO1 maintains pluripotency of primed human embryonic stem cells by promoting glycolysis. Stem Cells. https://doi.org/10.1002/stem.3044
Kropp, E. M., Oleson, B. J., Broniowska, K. A., Bhattacharya, S., Chadwick, A. C., Diers, A. R., Hu, Q., Sahoo, D., Hogg, N., Boheler, K. R., Corbett, J. A., & Gundry, R. L. (2015). Inhibition of an NAD(+) salvage pathway provides efficient and selective toxicity to human pluripotent stem cells. Stem Cells Translational Medicine. https://doi.org/10.5966/sctm.2014-0163
Wai, T., & Langer, T. (2016). Mitochondrial dynamics and metabolic regulation. Trends in Endocrinology and Metabolism. https://doi.org/10.1016/j.tem.2015.12.001
Prieto, J., Ponsoda, X., Izpisua Belmonte, J. C., & Torres, J. (2020). Mitochondrial dynamics and metabolism in induced pluripotency. Experimental Gerontology. https://doi.org/10.1016/j.exger.2020.110870
Hernandez-Aguilera, A., Rull, A., Rodriguez-Gallego, E., Riera-Borrull, M., Luciano-Mateo, F., Camps, J., Menendez, J. A., & Joven, J. (2013). Mitochondrial dysfunction: A basic mechanism in inflammation-related non-communicable diseases and therapeutic opportunities. Mediators of Inflammation. https://doi.org/10.1155/2013/135698
Suhr, S. T., Chang, E. A., Tjong, J., Alcasid, N., Perkins, G. A., Goissis, M. D., Ellisman, M. H., Perez, G. I., & Cibelli, J. B. (2010). Mitochondrial rejuvenation after induced pluripotency. PLoS One. https://doi.org/10.1371/journal.pone.0014095
St John, J. C., Ramalho-Santos, J., Gray, H. L., Petrosko, P., Rawe, V. Y., Navara, C. S., Simerly, C. R., & Schatten, G. P. (2005). The expression of mitochondrial DNA transcription factors during early cardiomyocyte in vitro differentiation from human embryonic stem cells. Cloning and Stem Cells. https://doi.org/10.1089/clo.2005.7.141
Cho, Y. M., Kwon, S., Pak, Y. K., Seol, H. W., Choi, Y. M., Park, D. J., Park, K. S., & Lee, H. K. (2006). Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochemical and Biophysical Research Communications. https://doi.org/10.1016/j.bbrc.2006.08.020
Spitkovsky, D., Sasse, P., Kolossov, E., Bottinger, C., Fleischmann, B. K., Hescheler, J., & Wiesner, R. J. (2004). Activity of complex III of the mitochondrial electron transport chain is essential for early heart muscle cell differentiation. The FASEB Journal. https://doi.org/10.1096/fj.03-0520fje
Park, J., Lee, S. B., Lee, S., Kim, Y., Song, S., Kim, S., Bae, E., Kim, J., Shong, M., Kim, J. M., & Chung, J. (2006). Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. https://doi.org/10.1038/nature04788
Okatsu, K., Oka, T., Iguchi, M., Imamura, K., Kosako, H., Tani, N., Kimura, M., Go, E., Koyano, F., Funayama, M., Shiba-Fukushima, K., Sato, S., Shimizu, H., Fukunaga, Y., Taniguchi, H., Komatsu, M., Hattori, N., Mihara, K., Tanaka, K., & Matsuda, N. (2012). PINK1 autophosphorylation upon membrane potential dissipation is essential for Parkin recruitment to damaged mitochondria. Nature Communications. https://doi.org/10.1038/ncomms2016
Ma, T., Li, J., Xu, Y., Yu, C., Xu, T., Wang, H., Liu, K., Cao, N., Nie, B. M., Zhu, S. Y., Xu, S., Li, K., Wei, W. G., Wu, Y., Guan, K. L., & Ding, S. (2015). Atg5-independent autophagy regulates mitochondrial clearance and is essential for iPSC reprogramming. Nature Cell Biology. https://doi.org/10.1038/ncb3256
Prieto, J., Leon, M., Ponsoda, X., Sendra, R., Bort, R., Ferrer-Lorente, R., Raya, A., Lopez-Garcia, C., & Torres, J. (2016). Early ERK1/2 activation promotes DRP1-dependent mitochondrial fission necessary for cell reprogramming. Nature Communications. https://doi.org/10.1038/ncomms11124
Wang, L., Xu, X., Jiang, C., Ma, G., Huang, Y., Zhang, H., Lai, Y., Wang, M., Ahmed, T., Lin, R., Guo, W., Luo, Z., Li, W., Zhang, M., Ward, C., Qian, M., Liu, B., Esteban, M. A., & Qin, B. (2020). mTORC1-PGC1 axis regulates mitochondrial remodeling during reprogramming. The FEBS Journal. https://doi.org/10.1111/febs.15024
Folmes, C. D., Dzeja, P. P., Nelson, T. J., & Terzic, A. (2012). Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell. https://doi.org/10.1016/j.stem.2012.10.002
Prigione, A., & andAdjaye, J. (2010). Modulation of mitochondrial biogenesis and bioenergetic metabolism upon in vitro and in vivo differentiation of human ES and iPS cells. The International Journal of Developmental Biology. https://doi.org/10.1387/ijdb.103198ap
Vessoni, A. T., Muotri, A. R., & Okamoto, O. K. (2012). Autophagy in stem cell maintenance and differentiation. Stem Cells and Development. https://doi.org/10.1089/scd.2011.0526
Santel, A., & andFuller, M.T. (2001). Control of mitochondrial morphology by a human mitofusin. Journal of Cell Science.
Santel, A., Frank, S., Gaume, B., Herrler, M., Youle, R. J., & Fuller, M. T. (2003). Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. Journal of Cell Science. https://doi.org/10.1242/jcs.00479
Eura, Y., Ishihara, N., Yokota, S., & Mihara, K. (2003). Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. Journal of Biochemistry. https://doi.org/10.1093/jb/mvg150
Olichon, A., Baricault, L., Gas, N., Guillou, E., Valette, A., Belenguer, P., & Lenaers, G. (2003). Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. The Journal of Biological Chemistry. https://doi.org/10.1074/jbc.C200677200
Cipolat, S., Martins de Brito, O., Dal Zilio, B., & Scorrano, L. (2004). OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.0407043101
Twig, G., Hyde, B., & Shirihai, O. S. (2008). Mitochondrial fusion, fission and autophagy as a quality control axis: The bioenergetic view. Biochimica et Biophysica Acta. https://doi.org/10.1016/j.bbabio.2008.05.001
Chen, H., & Chan, D. C. (2005). Emerging functions of mammalian mitochondrial fusion and fission. Human Molecular Genetics. https://doi.org/10.1093/hmg/ddi270
Kasahara, A., Cipolat, S., Chen, Y., Dorn 2nd, G. W., & Scorrano, L. (2013). Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and notch signaling. Science. https://doi.org/10.1126/science.1241359
Son, M. J., Kwon, Y., Son, M. Y., Seol, B., Choi, H. S., Ryu, S. W., Choi, C., & Cho, Y. S. (2015). Mitofusins deficiency elicits mitochondrial metabolic reprogramming to pluripotency. Cell Death and Differentiation. https://doi.org/10.1038/cdd.2015.43
Wang, L., Ye, X., Zhao, Q., Zhou, Z., Dan, J., Zhu, Y., Chen, Q., & Liu, L. (2014). Drp1 is dispensable for mitochondria biogenesis in induction to pluripotency but required for differentiation of embryonic stem cells. Stem Cells and Development. https://doi.org/10.1089/scd.2014.0059
Kim, H. J., Shaker, M. R., Cho, B., Cho, H. M., Kim, H., Kim, J. Y., & Sun, W. (2015). Dynamin-related protein 1 controls the migration and neuronal differentiation of subventricular zone-derived neural progenitor cells. Scientific Reports. https://doi.org/10.1038/srep15962
Son, M. Y., Choi, H., Han, Y. M., & Cho, Y. S. (2013). Unveiling the critical role of REX1 in the regulation of human stem cell pluripotency. Stem Cells. https://doi.org/10.1002/stem.1509
Hoque, A., Sivakumaran, P., Bond, S. T., Ling, N. X. Y., Kong, A. M., Scott, J. W., Bandara, N., Hernandez, D., Liu, G. S., Wong, R. C. B., Ryan, M. T., Hausenloy, D. J., Kemp, B. E., Oakhill, J. S., Drew, B. G., Pebay, A., & Lim, S. Y. (2018). Mitochondrial fission protein Drp1 inhibition promotes cardiac mesodermal differentiation of human pluripotent stem cells. Cell Death Discov. https://doi.org/10.1038/s41420-018-0042-9
Xu, C., Bailly-Maitre, B., & Reed, J. C. (2005). Endoplasmic reticulum stress: Cell life and death decisions. The Journal of Clinical Investigation. https://doi.org/10.1172/JCI26373
Ulianich, L., Garbi, C., Treglia, A. S., Punzi, D., Miele, C., Raciti, G. A., Beguinot, F., Consiglio, E., & Di Jeso, B. (2008). ER stress is associated with dedifferentiation and an epithelial-to-mesenchymal transition-like phenotype in PC Cl3 thyroid cells. Journal of Cell Science. https://doi.org/10.1242/jcs.017202
Saran, M., & Bors, W. (1990). Radical reactions in vivo--an overview. Radiation and Environmental Biophysics. https://doi.org/10.1007/BF01210406
Oka, O. B., & Bulleid, N. J. (2013). Forming disulfides in the endoplasmic reticulum. Biochimica et Biophysica Acta. https://doi.org/10.1016/j.bbamcr.2013.02.007
Marsboom, G., Zhang, G. F., Pohl-Avila, N., Zhang, Y., Yuan, Y., Kang, H., Hao, B., Brunengraber, H., Malik, A. B., & Rehman, J. (2016). Glutamine metabolism regulates the pluripotency transcription factor OCT4. Cell Reports. https://doi.org/10.1016/j.celrep.2016.05.089
Nishitoh, H. (2012). CHOP is a multifunctional transcription factor in the ER stress response. Journal of Biochemistry. https://doi.org/10.1093/jb/mvr143
Goldenberg-Cohen, N., Raiter, A., Gaydar, V., Dratviman-Storobinsky, O., Goldstein, T., Weizman, A., & Hardy, B. (2012). Peptide-binding GRP78 protects neurons from hypoxia-induced apoptosis. Apoptosis. https://doi.org/10.1007/s10495-011-0678-x
Caballano-Infantes, E., Terron-Bautista, J., Beltran-Povea, A., Cahuana, G. M., Soria, B., Nabil, H., Bedoya, F. J., & Tejedo, J. R. (2017). Regulation of mitochondrial function and endoplasmic reticulum stress by nitric oxide in pluripotent stem cells. World J Stem Cells. https://doi.org/10.4252/wjsc.v9.i2.26
Luo, S., Mao, C., Lee, B., & Lee, A. S. (2006). GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Molecular and Cellular Biology. https://doi.org/10.1128/MCB.00779-06
Varum, S., Rodrigues, A. S., Moura, M. B., Momcilovic, O., Easley, C. A. T., Ramalho-Santos, J., Van Houten, B., & Schatten, G. (2011). Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. https://doi.org/10.1371/journal.pone.0020914
Kratochvilova, K., Moran, L., Padourova, S., Stejskal, S., Tesarova, L., Simara, P., Hampl, A., Koutna, I., & Vanhara, P. (2016). The role of the endoplasmic reticulum stress in stemness, pluripotency and development. European Journal of Cell Biology. https://doi.org/10.1016/j.ejcb.2016.02.002
Bianco, C., Rangel, M. C., Castro, N. P., Nagaoka, T., Rollman, K., Gonzales, M., & Salomon, D. S. (2010). Role of Cripto-1 in stem cell maintenance and malignant progression. The American Journal of Pathology. https://doi.org/10.2353/ajpath.2010.100102
Ding, J., Yang, L., Yan, Y. T., Chen, A., Desai, N., Wynshaw-Boris, A., & Shen, M. M. (1998). Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. https://doi.org/10.1038/27215
Miharada, K., Karlsson, G., Rehn, M., Rorby, E., Siva, K., Cammenga, J., & Karlsson, S. (2011). Cripto regulates hematopoietic stem cells as a hypoxic-niche-related factor through cell surface receptor GRP78. Cell Stem Cell. https://doi.org/10.1016/j.stem.2011.07.016
Babaie, Y., Herwig, R., Greber, B., Brink, T. C., Wruck, W., Groth, D., Lehrach, H., Burdon, T., & Adjaye, J. (2007). Analysis of Oct4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cells. Stem Cells. https://doi.org/10.1634/stemcells.2006-0426
Loh, Y. H., Wu, Q., Chew, J. L., Vega, V. B., Zhang, W., Chen, X., Bourque, G., George, J., Leong, B., Liu, J., Wong, K. Y., Sung, K. W., Lee, C. W., Zhao, X. D., Chiu, K. P., Lipovich, L., Kuznetsov, V. A., Robson, P., Stanton, L. W., et al. (2006). The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nature Genetics. https://doi.org/10.1038/ng1760
Bianco, C., Cotten, C., Lonardo, E., Strizzi, L., Baraty, C., Mancino, M., Gonzales, M., Watanabe, K., Nagaoka, T., Berry, C., Arai, A. E., Minchiotti, G., & Salomon, D. S. (2009). Cripto-1 is required for hypoxia to induce cardiac differentiation of mouse embryonic stem cells. The American Journal of Pathology. https://doi.org/10.2353/ajpath.2009.090218
Chen, A., Muzzio, I. A., Malleret, G., Bartsch, D., Verbitsky, M., Pavlidis, P., Yonan, A. L., Vronskaya, S., Grody, M. B., Cepeda, I., Gilliam, T. C., & Kandel, E. R. (2003). Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. https://doi.org/10.1016/s0896-6273(03)00501-4
Del Vecchio, C. A., Feng, Y., Sokol, E. S., Tillman, E. J., Sanduja, S., Reinhardt, F., & Gupta, P. B. (2014). De-differentiation confers multidrug resistance via noncanonical PERK-Nrf2 signaling. PLoS Biology. https://doi.org/10.1371/journal.pbio.1001945
Cullinan, S. B., Zhang, D., Hannink, M., Arvisais, E., Kaufman, R. J., & Diehl, J. A. (2003). Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Molecular and Cellular Biology. https://doi.org/10.1128/mcb.23.20.7198-7209.2003
Ogawa, Y., Saito, Y., Nishio, K., Yoshida, Y., Ashida, H., & Niki, E. (2008). Gamma-tocopheryl quinone, not alpha-tocopheryl quinone, induces adaptive response through up-regulation of cellular glutathione and cysteine availability via activation of ATF4. Free Radical Research. https://doi.org/10.1080/10715760802277396
Sato, H., Nomura, S., Maebara, K., Sato, K., Tamba, M., & Bannai, S. (2004). Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochemical and Biophysical Research Communications. https://doi.org/10.1016/j.bbrc.2004.10.009
Zinszner, H., Kuroda, M., Wang, X., Batchvarova, N., Lightfoot, R. T., Remotti, H., Stevens, J. L., & Ron, D. (1998). CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes & Development. https://doi.org/10.1101/gad.12.7.982
Tapia-Limonchi, R., Diaz, I., Cahuana, G. M., Bautista, M., Martin, F., Soria, B., Tejedo, J. R., & Bedoya, F. J. (2014). Impact of exposure to low concentrations of nitric oxide on protein profile in murine and human pancreatic islet cells. Islets. https://doi.org/10.1080/19382014.2014.995997
Blanco-Gelaz, M. A., Suarez-Alvarez, B., Ligero, G., Sanchez, L., Vidal-Castineira, J. R., Coto, E., Moore, H., Menendez, P., & Lopez-Larrea, C. (2010). Endoplasmic reticulum stress signals in defined human embryonic stem cell lines and culture conditions. Stem Cell Reviews and Reports. https://doi.org/10.1007/s12015-010-9135-4
Puente, L. G., Borris, D. J., Carriere, J. F., Kelly, J. F., & Megeney, L. A. (2006). Identification of candidate regulators of embryonic stem cell differentiation by comparative phosphoprotein affinity profiling. Molecular & Cellular Proteomics. https://doi.org/10.1074/mcp.M500166-MCP200
Canová, N. K., Kmoníčková, E., Martínek, J., Zídek, Z., & Farghali, H. (2007). Thapsigargin, a selective inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases, modulates nitric oxide production and cell death of primary rat hepatocytes in culture. Cell Biology and Toxicology. https://doi.org/10.1007/s10565-007-0185-6
Ben-David, U., Gan, Q. F., Golan-Lev, T., Arora, P., Yanuka, O., Oren, Y. S., Leikin-Frenkel, A., Graf, M., Garippa, R., Boehringer, M., Gromo, G., & Benvenisty, N. (2013). Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. https://doi.org/10.1016/j.stem.2012.11.015
Chen, G., Xu, X., Zhang, L., Fu, Y., Wang, M., Gu, H., & Xie, X. (2014). Blocking autocrine VEGF signaling by sunitinib, an anti-cancer drug, promotes embryonic stem cell self-renewal and somatic cell reprogramming. Cell Research. https://doi.org/10.1038/cr.2014.112
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T., & Mori, K. (2001). XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. https://doi.org/10.1016/s0092-8674(01)00611-0
Liu, L., Liu, C., Zhong, Y., Apostolou, A., & Fang, S. (2012). ER stress response during the differentiation of H9 cells induced by retinoic acid. Biochemical and Biophysical Research Communications. https://doi.org/10.1016/j.bbrc.2011.12.026
Simic, M. S., Moehle, E. A., Schinzel, R. T., Lorbeer, F. K., Halloran, J. J., Heydari, K., Sanchez, M., Jullie, D., Hockemeyer, D., & Dillin, A. (2019). Transient activation of the UPR(ER) is an essential step in the acquisition of pluripotency during reprogramming. Science Advances. https://doi.org/10.1126/sciadv.aaw0025
Brambrink, T., Foreman, R., Welstead, G. G., Lengner, C. J., Wernig, M., Suh, H., & Jaenisch, R. (2008). Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. https://doi.org/10.1016/j.stem.2008.01.004
Kornmann, B., & Walter, P. (2010). ERMES-mediated ER-mitochondria contacts: Molecular hubs for the regulation of mitochondrial biology. Journal of Cell Science. https://doi.org/10.1242/jcs.058636
Raturi, A., & Simmen, T. (2013). Where the endoplasmic reticulum and the mitochondrion tie the knot: The mitochondria-associated membrane (MAM). Biochimica et Biophysica Acta. https://doi.org/10.1016/j.bbamcr.2012.04.013
Hansford, R. G. (1985). Relation between mitochondrial calcium transport and control of energy metabolism. Reviews of Physiology, Biochemistry and Pharmacology. https://doi.org/10.1007/BFb0034084
Csordas, G., Varnai, P., Golenar, T., Roy, S., Purkins, G., Schneider, T. G., Balla, T., & Hajnoczky, G. (2010). Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Molecular Cell. https://doi.org/10.1016/j.molcel.2010.06.029
Gellerich, F. N., Gizatullina, Z., Trumbeckaite, S., Nguyen, H. P., Pallas, T., Arandarcikaite, O., Vielhaber, S., Seppet, E., & Striggow, F. (2010). The regulation of OXPHOS by extramitochondrial calcium. Biochimica et Biophysica Acta. https://doi.org/10.1016/j.bbabio.2010.02.005
Szabadkai, G., Bianchi, K., Varnai, P., De Stefani, D., Wieckowski, M. R., Cavagna, D., Nagy, A. I., Balla, T., & Rizzuto, R. (2006). Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. The Journal of Cell Biology. https://doi.org/10.1083/jcb.200608073
Csordas, G., Renken, C., Varnai, P., Walter, L., Weaver, D., Buttle, K. F., Balla, T., Mannella, C. A., & Hajnoczky, G. (2006). Structural and functional features and significance of the physical linkage between ER and mitochondria. The Journal of Cell Biology. https://doi.org/10.1083/jcb.200604016
Simmen, T., Aslan, J. E., Blagoveshchenskaya, A. D., Thomas, L., Wan, L., Xiang, Y., Feliciangeli, S. F., Hung, C. H., Crump, C. M., & Thomas, G. (2005). PACS-2 controls endoplasmic reticulum-mitochondria communication and bid-mediated apoptosis. The EMBO Journal. https://doi.org/10.1038/sj.emboj.7600559
Golshani-Hebroni, S. G., & Bessman, S. P. (1997). Hexokinase binding to mitochondria: A basis for proliferative energy metabolism. Journal of Bioenergetics and Biomembranes. https://doi.org/10.1023/a:1022442629543
Majewski, N., Nogueira, V., Robey, R. B., & Hay, N. (2004). Akt inhibits apoptosis downstream of BID cleavage via a glucose-dependent mechanism involving mitochondrial hexokinases. Molecular and Cellular Biology. https://doi.org/10.1128/mcb.24.2.730-740.2004
Sheridan, C., & Martin, S. J. (2010). Mitochondrial fission/fusion dynamics and apoptosis. Mitochondrion. https://doi.org/10.1016/j.mito.2010.08.005
Friedman, J. R., Lackner, L. L., West, M., DiBenedetto, J. R., Nunnari, J., & Voeltz, G. K. (2011). ER tubules mark sites of mitochondrial division. Science. https://doi.org/10.1126/science.1207385
de Brito, O. M., & Scorrano, L. (2008). Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. https://doi.org/10.1038/nature07534
Delmotte, P., & Sieck, G. C. (2019). Endoplasmic reticulum stress and mitochondrial function in airway smooth muscle. Frontiers in Cell and Development Biology. https://doi.org/10.3389/fcell.2019.00374
Youngman, M. J., Hobbs, A. E., Burgess, S. M., Srinivasan, M., & Jensen, R. E. (2004). Mmm2p, a mitochondrial outer membrane protein required for yeast mitochondrial shape and maintenance of mtDNA nucleoids. The Journal of Cell Biology. https://doi.org/10.1083/jcb.200308012
Lewis, S. C., Uchiyama, L. F., & Nunnari, J. (2016). ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science. https://doi.org/10.1126/science.aaf5549
Basso, V., Marchesan, E., Peggion, C., Chakraborty, J., von Stockum, S., Giacomello, M., Ottolini, D., Debattisti, V., Caicci, F., Tasca, E., Pegoraro, V., Angelini, C., Antonini, A., Bertoli, A., Brini, M., & Ziviani, E. (2018). Regulation of ER-mitochondria contacts by Parkin via Mfn2. Pharmacological Research. https://doi.org/10.1016/j.phrs.2018.09.006
McLelland, G. L., Goiran, T., Yi, W., Dorval, G., Chen, C. X., Lauinger, N. D., Krahn, A. I., Valimehr, S., Rakovic, A., Rouiller, I., Durcan, T. M., Trempe, J. F., & Fon, E. A. (2018). Mfn2 ubiquitination by PINK1/parkin gates the p97-dependent release of ER from mitochondria to drive mitophagy. Elife. https://doi.org/10.7554/eLife.32866
Author information
Authors and Affiliations
Contributions
Babaei-Abraki: conceptualization, literature search, original draft writing, and editing. Karamali: contributed to the study conception, design, and editing. Nasr-Esfahani: critically reviewed the manuscript, supervision it, and approved the final version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Babaei-Abraki, S., Karamali, F. & Nasr-Esfahani, M.H. The Role of Endoplasmic Reticulum and Mitochondria in Maintaining Redox Status and Glycolytic Metabolism in Pluripotent Stem Cells. Stem Cell Rev and Rep 18, 1789–1808 (2022). https://doi.org/10.1007/s12015-022-10338-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10338-8