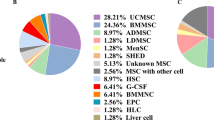
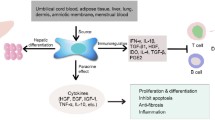
Abstract
The whole liver transplantation is the most effective treatment for end-stage fibrosis. However, the lack of available donors, immune rejection and total cost of surgery remain as the key challenges in advancing liver fibrosis therapeutics. Due to the multi-differentiation and low immunogenicity of stem cells, treatment of liver fibrosis with stem cells has been considered as a valuable new therapeutic modality. The pathological progression of liver fibrosis is closely related to the changes in the activities of intrahepatic cells. Damaged hepatocytes, activated Kupffer cells and other inflammatory cells lead to hepatic stellate cells (HSCs) activation, further promoting apoptosis of damaged hepatocytes, while stem cells can work on fibrosis-related intrahepatic cells through relevant transduction pathways. Herein, this article elucidates the phenomena and the mechanisms of the crosstalk between various types of stem cells and intrahepatic cells including HSCs and hepatocytes in the treatment of liver fibrosis. Then, the important influences of chemical compositions, mechanical properties and blood flow on liver fibrosis models with stem cell treatment are emphasized. Clinical trials on stem cell-based therapy for liver fibrosis are also briefly summarized. Finally, continuing challenges and future directions of stem cell-based therapy for hepatic fibrosis are discussed. In short, stem cells play an important advantage and have a great potential in treating liver fibrosis by interacting with intrahepatic cells. Clarifying how stem cells interact with intrahepatic cells to change the progression of liver fibrosis is of great significance for a deeper understanding of liver fibrosis mechanisms and targeted therapy.
Graphic Abstract





Similar content being viewed by others
References
Molokanova, O., Schönig, K., Weng, S. Y., Wang, X., Bros, M., Diken, M., et al. (2018). Inducible knockdown of procollagen I protects mice from liver fibrosis and leads to dysregulated matrix genes and attenuated inflammation. Matrix Biology, 66, 34–49
Carvalho, A. B., Quintanilha, L. F., Dias, J. V., Paredes, B. D., Mannheimer, E. G., Carvalho, F. G., et al. (2008). Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells, 26(5), 1307–1314
Mederacke, I., Hsu, C. C., Troeger, J. S., Huebener, P., Mu, X., Dapito, D. H., et al. (2013). Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nature Communications, 4, 2823
Seki, A., Sakai, Y., Komura, T., Nasti, A., Yoshida, K., Higashimoto, M., et al. (2013). Adipose tissue-derived stem cells as a regenerative therapy for a mouse steatohepatitis-induced cirrhosis model. Hepatology, 58(3), 1133–1142
Qian, H., Deng, X., Huang, Z. W., Wei, J., Ding, C. H., Feng, R. X., et al. (2015). An HNF1α-regulated feedback circuit modulates hepatic fibrogenesis via the crosstalk between hepatocytes and hepatic stellate cells. Cell Research, 25(8), 930–945
Liu, L., Yannam, G. R., Nishikawa, T., Yamamoto, T., Basma, H., Ito, R., et al. (2012). The microenvironment in hepatocyte regeneration and function in rats with advanced cirrhosis. Hepatology, 55(5), 1529–1539
Pingitore, P., Sasidharan, K., Ekstrand, M., Prill, S., Lindén, D., & Romeo, S. (2019). Human multilineage 3D spheroids as a model of liver steatosis and fibrosis. International Journal of Molecular Sciences, 20(7), 1629
Jones, R., Lebkowski, J., & McNiece, I. (2010). Stem cells. Biology of Blood and Marrow Transplantation, 16(1 Suppl), S115–S118
Cai, X., Wang, J., Wang, J., Zhou, Q., Yang, B., He, Q., et al. (2020). Intercellular crosstalk of hepatic stellate cells in liver fibrosis: New insights into therapy. Pharmacological Research, 155, 104720
Najimi, M., Berardis, S., El-Kehdy, H., Rosseels, V., Evraerts, J., Lombard, C., et al. (2017). Human liver mesenchymal stem/progenitor cells inhibit hepatic stellate cell activation: in vitro and in vivo evaluation. Stem Cell Research & Therapy, 8(1), 131
Park, M., Kim, Y. H., Woo, S. Y., Lee, H. J., Yu, Y., Kim, H. S., et al. (2015). Tonsil-derived mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in mice via autophagy activation. Scientific Reports, 5, 8616
Zhang, Y., Li, Y., Zhang, L., Li, J., & Zhu, C. (2018). Mesenchymal stem cells: potential application for the treatment of hepatic cirrhosis. Stem Cell Research & Therapy, 9(1), 59
Yang, X., Han, Z. P., Zhang, S. S., Zhu, P. X., Hao, C., Fan, T. T., et al. (2014). Chronic restraint stress decreases the repair potential from mesenchymal stem cells on liver injury by inhibiting TGF-β1 generation. Cell Death and Disease, 5(6), e1308
Jang, Y. O., Jun, B. G., Baik, S. K., Kim, M. Y., & Kwon, S. O. (2015). Inhibition of hepatic stellate cells by bone marrow-derived mesenchymal stem cells in hepatic fibrosis. Clinical and Molecular Hepatology, 21(2), 141–149
Huang, B., Cheng, X., Wang, H., Huang, W., la Ga Hu, Z., Wang, D., et al. (2016). Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. Journal of Translational Medicine, 14, 45
Ribot, E. J., Gaudet, J. M., Chen, Y., Gilbert, K. M., & Foster, P. J. (2014). In vivo MR detection of fluorine-labeled human MSC using the bSSFP sequence. International Journal of Nanomedicine, 9, 1731-1739
Eggenhofer, E., Luk, F., Dahlke, M. H., & Hoogduijn, M. J. (2014). The life and fate of mesenchymal stem cells. Frontiers in Immunology, 5, 148
Chen, Y., Xiang, L. X., Shao, J. Z., Pan, R. L., Wang, Y. X., Dong, X. J., et al. (2010). Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. Journal of Cellular and Molecular Medicine, 14(6B), 1494–1508
Lou, G., Yang, Y., Liu, F., Ye, B., Chen, Z., Zheng, M., et al. (2017). MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. Journal of Cellular and Molecular Medicine, 21(11), 2963–2973
Al Ghrbawy, N. M., Afify, R. A., Dyaa, N., & El Sayed, A. A. (2016). Differentiation of bone marrow: derived mesenchymal stem cells into hepatocyte-like cells. Indian J Hematol Blood Transfus, 32(3), 276–283
Ewida, S. F., Abdou, A. G., El-Rasol Elhosary, A. A., & El-Ghane Metawe, S. A. (2017). Hepatocyte-like versus mesenchymal stem cells in CCl4-induced liver fibrosis. Applied Immunohistochemistry & Molecular Morphology, 25(10), 736–745
Kim, M. D., Kim, S. S., Cha, H. Y., Jang, S. H., Chang, D. Y., Kim, W., et al. (2014). Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Experimental & Molecular Medicine, 46(8), e110
Li, C., Kong, Y., Wang, H., Wang, S., Yu, H., Liu, X., et al. (2009). Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. Journal of Hepatology, 50(6), 1174–1183
Russo, F. P., Alison, M. R., Bigger, B. W., Amofah, E., Florou, A., Amin, F., et al. (2006). The bone marrow functionally contributes to liver fibrosis. Gastroenterology, 130(6), 1807–1821
di Bonzo, L. V., Ferrero, I., Cravanzola, C., Mareschi, K., Rustichell, D., Novo, E., et al. (2008). Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: engraftment and hepatocyte differentiation versus profibrogenic potential. Gut, 57(2), 223–231
Overi, D., Carpino, G., Franchitto, A., Onori, P., & Gaudio, E. (2020). Hepatocyte injury and hepatic stem cell niche in the progression of non-alcoholic steatohepatitis. Cells, 9(3), 590
Hao, T., Chen, J., Zhi, S., Zhang, Q., Chen, G., & Yu, F. (2017). Comparison of bone marrow-vs. adipose tissue-derived mesenchymal stem cells for attenuating liver fibrosis. Experimental and Therapeutic Medicine, 14(6), 5956–5964
Meier, R. P., Mahou, R., Morel, P., Meyer, J., Montanari, E., Muller, Y. D., et al. (2015). Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. Journal of Hepatology, 62(3), 634–641
Rambhatla, L., Chiu, C. P., Kundu, P., Peng, Y., & Carpenter, M. K. (2003). Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplantation, 12(1), 1–11
Takayama, K., Inamura, M., Kawabata, K., Katayama, K., Higuchi, M., Tashiro, K., et al. (2012). Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4α transduction. Molecular Therapy, 20(1), 127–137
Tolosa, L., Caron, J., Hannoun, Z., Antoni, M., López, S., Burks, D., et al. (2015). Transplantation of hESC-derived hepatocytes protects mice from liver injury. Stem Cell Research & Therapy, 6, 246
Yamamoto, H., Quinn, G., Asari, A., Yamanokuchi, H., Teratani, T., Terada, M., et al. (2003). Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology, 37(5), 983–993
Teratani, T., Yamamoto, H., Aoyagi, K., Sasaki, H., Asari, A., Quinn, G., et al. (2005). Direct hepatic fate specification from mouse embryonic stem cells. Hepatology, 41(4), 836–846
Woo, D. H., Kim, S. K., Lim, H. J., Heo, J., Park, H. S., Kang, G. Y., et al. (2012). Direct and indirect contribution of human embryonic stem cell-derived hepatocyte-like cells to liver repair in mice. Gastroenterology, 142(3), 602–611
Li, Q., Hutchins, A. P., Chen, Y., Li, S., Shan, Y., Liao, B., et al. (2017). A sequential EMT-MET mechanism drives the differentiation of human embryonic stem cells towards hepatocytes. Nature Communications, 8, 15166
Liu, H., Kim, Y., Sharkis, S., Marchionni, L., & Jang, Y. Y. (2011). In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Science Translational Medicine, 3(82), 8239
Dianat, N., Dubois-Pot-Schneider, H., Steichen, C., Desterke, C., Leclerc, P., Raveux, A., et al. (2014). Generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology, 60(2), 700–714
Li, X., Chen, R., Kemper, S., & Brigstock, D. R. (2020). Extracellular vesicles from hepatocytes are therapeutic for toxin-mediated fibrosis and gene expression in the liver. Frontiers in Cell and Developmental Biology, 7, 368
Kowal, J., Arras, G., Colombo, M., Jouve, M., Morath, J. P., Primdal-Bengtson, B., et al. (2016). Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proceedings of the National Academy of Sciences of the United States of America, 113(8), E968–E977
Povero, D., Pinatel, E. M., Leszczynska, A., Goyal, N. P., Nishio, T., Kim, J., et al. (2019). Human induced pluripotent stem cell-derived extracellular vesicles reduce hepatic stellate cell activation and liver fibrosis. JCI Insight, 5(14), e125652
Rengasamy, M., Singh, G., Fakharuzi, N. A., Siddikuzzaman, Balasubramanian, S., Swamynathan, P., et al. (2017). Transplantation of human bone marrow mesenchymal stromal cells reduces liver fibrosis more effectively than Wharton’s jelly mesenchymal stromal cells. Stem Cell Research & Therapy, 8(1), 143
Rong, X., Liu, J., Yao, X., Jiang, T., Wang, Y., & Xie, F. (2019). Human bone marrow mesenchymal stem cells-derived exosomes alleviate liver fibrosis through the Wnt/β-catenin pathway. Stem Cell Research & Therapy, 10(1), 98
Li, T., Yan, Y., Wang, B., Qian, H., Zhang, X., Shen, L., et al. (2013). Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev, 22(6), 845–854
Zhang, Y., Xu, J., Liu, S., Lim, M., Zhao, S., Cui, K., et al. (2019). Embryonic stem cell-derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells. Theranostics, 9(23), 6976–6990
Mardpour, S., Ghanian, M. H., Sadeghi-Abandansari, H., Mardpour, S., Nazari, A., Shekari, F., et al. (2019). Hydrogel-mediated sustained systemic delivery of mesenchymal stem cell-derived extracellular vesicles improves hepatic regeneration in chronic liver failure. ACS Applied Materials & Interfaces, 11(41), 37421–37433
Pan, R. L., Wang, P., Xiang, L. X., & Shao, J. Z. (2011). Delta-like 1 serves as a new target and contributor to liver fibrosis down-regulated by mesenchymal stem cell transplantation. The Journal of Biological Chemistry, 286(14), 12340–12348
Huang, C. K., Lee, S. O., Lai, K. P., Ma, W. L., Lin, T. H., Tsai, M. Y., et al. (2013). Targeting androgen receptor in bone marrow mesenchymal stem cells leads to better transplantation therapy efficacy in liver cirrhosis. Hepatology, 57(4), 1550–1563
An, S. Y., Jang, Y. J., Lim, H. J., Han, J., Lee, J., Lee, G., et al. (2017). Milk fat globule-EGF factor 8, secreted by mesenchymal stem cells, protects against liver fibrosis in mice. Gastroenterology, 152(5), 1174–1186
Dooley, S., Hamzavi, J., Breitkopf, K., Wiercinska, E., Said, H. M., Lorenzen, J., et al. (2003). Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology, 125(1), 178–191
Derynck, R., & Zhang, Y. E. (2003). Smad-dependent and Smad-independent pathways in TGF-beta family signaling. Nature, 425(6958), 577–584
Zhang, L. T., Fang, X. Q., Chen, Q. F., Chen, H., Xiao, P., Peng, X. B., et al. (2015). Bone marrow-derived mesenchymal stem cells inhibit the proliferation of hepatic stellate cells by inhibiting the transforming growth factor β pathway. Molecular Medicine Reports, 12(5), 7227–7232
Qu, Y., Zhang, Q., Cai, X., Li, F., Ma, Z., Xu, M., et al. (2017). Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. Journal of Cellular and Molecular Medicine, 21(10), 2491–2502
Contreras-Jurado, C., Alonso-Merino, E., Saiz-Ladera, C., Valiño, A. J., Regadera, J., Alemany, S., et al. (2016). The thyroid hormone receptors inhibit hepatic interleukin-6 signaling during endotoxemia. Scientific Reports, 6, 30990
Qiao, H., Zhou, Y., Qin, X., Cheng, J., He, Y., & Jiang, Y. (2018). NADPH oxidase signaling pathway mediates mesenchymal stem cell-induced inhibition of hepatic stellate cell activation. Stem Cells International, 2018, 1239143
Lin, N., Tang, Z., Deng, M., Zhong, Y., Lin, J., Yang, X., et al. (2008). Hedgehog-mediated paracrine interaction between hepatic stellate cells and marrow-derived mesenchymal stem cells. Biochemical and Biophysical Research Communications, 372(1), 260–265
Wang, P. P., Xie, D. Y., Liang, X. J., Peng, L., Zhang, G. L., Ye, Y. N., et al. (2012). HGF and direct mesenchymal stem cells contact synergize to inhibit hepatic stellate cells activation through TLR4/NF-kB pathway. PLoS One, 7(8), e43408
Chillakuri, C. R., Sheppard, D., Lea, S. M., & Handford, P. A. (2012). Notch receptor-ligand binding and activation: insights from molecular studies. Seminars in Cell & Developmental Biology, 23(4), 421–428
Hurlbut, G. D., Kankel, M. W., Lake, R. J., & Artavanis-Tsakonas, S. (2007). Crossing paths with Notch in the hyper-network. Current Opinion in Cell Biology, 19(2), 166–175
Chen, S., Xu, L., Lin, N., Pan, W., Hu, K., & Xu, R. (2011). Activation of Notch1 signaling by marrow-derived mesenchymal stem cells through cell-cell contact inhibits proliferation of hepatic stellate cells. Life Sciences, 89(25–26), 975–981.
Zhang, J., Corciulo, C., Liu, H., Wilder, T., Ito, M., & Cronstein, B. (2017). Adenosine A2a receptor blockade diminishes Wnt/β-Catenin signaling in a murine model of bleomycin-induced dermal fibrosis. The American Journal of Pathology, 187(9), 1935–1944
Rahmani, F., Avan, A., Hashemy, S. I., & Hassanian, S. M. (2018). Role of Wnt/β-catenin signaling regulatory microRNAs in the pathogenesis of colorectal cancer. Journal of Cellular Physiology, 233(2), 811–817
So, J., Khaliq, M., Evason, K., Ninov, N., Martin, B. L., Stainier, D. Y. R., et al. (2018). Wnt/β-catenin signaling controls intrahepatic biliary network formation in zebrafish by regulating notch activity. Hepatology, 67(6), 2352–2366
Nakhaei-Rad, S., Nakhaeizadeh, H., Götze, S., Kordes, C., Sawitza, I., Hoffmann, M. J., et al. (2016). The role of embryonic stem cell-expressed RAS (ERAS) in the maintenance of quiescent hepatic stellate cells. The Journal of Biological Chemistry, 291(16), 8399–8413
Zhang, Z., Zhou, X., Shen, H., Wang, D., & Wang, Y. (2009). Phosphorylated ERK is a potential predictor of sensitivity to sorafenib when treating hepatocellular carcinoma: evidence from an in vitro study. BMC Medicine, 7, 41
Simonetto, D. A., Yang, H. Y., Yin, M., de Assuncao, T. M., Kwon, J. H., Hilscher, M., et al. (2015). Chronic passive venous congestion drives hepatic fibrogenesis via sinusoidal thrombosis and mechanical forces. Hepatology, 61(2), 648–659
Ehling, J., Bartneck, M., Wei, X., Gremse, F., Fech, V., Möckel, D., et al. (2014). CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut, 63(12), 1960–1971
Yanagawa, T., Sumiyoshi, H., Higashi, K., Nakao, S., Higashiyama, R., Fukumitsu, H., et al. (2019). Identification of a novel bone marrow cell-derived accelerator of fibrotic liver regeneration through mobilization of hepatic progenitor cells in mice. Stem Cells, 37(1), 89–101
Liu, Y., Yang, X., Jing, Y., Zhang, S., Zong, C., Jiang, J., et al. (2015). Contribution and mobilization of mesenchymal stem cells in a mouse model of carbon tetrachloride-induced liver fibrosis. Scientific Reports, 5, 17762
Zhang, L. T., Peng, X. B., Fang, X. Q., Li, J. F., Chen, H., & Mao, X. R. (2018). Human umbilical cord mesenchymal stem cells inhibit proliferation of hepatic stellate cells in vitro. International Journal of Molecular Medicine, 41(5), 2545–2552
De Minicis, S., Seki, E., Uchinami, H., Kluwe, J., Zhang, Y., Brenner, D. A., et al. (2007). Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology, 132(5), 1937–1946
Pampaloni, F., Reynaud, E. G., & Stelzer, E. H. (2007). The third dimension bridges the gap between cell culture and live tissue. Nature Reviews. Molecular Cell Biology, 8(10), 839–845
Leite, S. B., Roosens, T., El Taghdouini, A., Mannaerts, I., Smout, A. J., Najimi, M., et al. (2016). Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials, 78, 1–10
Olinga, P., & Schuppan, D. (2013). Precision-cut liver slices: a tool to model the liver ex vivo. Journal of Hepatology, 58(6), 1252–1253
Wang, S., Wang, X., Tan, Z., Su, Y., Liu, J., Chang, M., et al. (2019). Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Research, 29(12), 1009–1026
Coll, M., Perea, L., Boon, R., Leite, S. B., Vallverdú, J., Mannaerts, I., et al. (2018). Generation of hepatic stellate cells from human pluripotent stem cells enables in vitro modeling of liver fibrosis. Cell Stem Cell, 23(1), 101–1137
Morales-Ibanez, O., Affò, S., Rodrigo-Torres, D., Blaya, D., Millán, C., Coll, M., et al. (2016). Kinase analysis in alcoholic hepatitis identifies p90RSK as a potential mediator of liver fibrogenesis. Gut, 65(5), 840–851
Novoseletskaya, E. S., Grigorieva, O. A., Efimenko, A. Y., & Kalinina, N. I. (2019). Extracellular matrix in the regulation of stem cell differentiation. Biochemistry (Mosc), 84(3), 232–240
Ji, R., Zhang, N., You, N., Li, Q., Liu, W., Jiang, N., et al. (2012). The differentiation of MSCs into functional hepatocyte-like cells in a liver biomatrix scaffold and their transplantation into liver-fibrotic mice. Biomaterials, 33(35), 8995–9008
Iwakiri, Y. (2014). Pathophysiology of portal hypertension. Clinics in Liver Disease, 18(2), 281–291
Brückner, S., Zipprich, A., Hempel, M., Thonig, A., Schwill, F., Roderfeld, M., et al. (2017). Improvement of portal venous pressure in cirrhotic rat livers by systemic treatment with adipose tissue-derived mesenchymal stromal cells. Cytotherapy, 19(12), 1462–1473
Friedman, S. L. (2000). Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. The Journal of Biological Chemistry, 275(4), 2247–2250
Suk, K. T., Yoon, J. H., Kim, M. Y., Kim, C. W., Kim, J. K., Park, H., et al. (2016). Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology, 64(6), 2185–2197
Uygun, B. E., Soto-Gutierrez, A., Yagi, H., Izamis, M. L., Guzzardi, M. A., Shulman, C., et al. (2010). Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nature Medicine, 16(7), 814–820
Kojima, H., Yasuchika, K., Fukumitsu, K., Ishii, T., Ogiso, S., Miyauchi, Y., et al. (2018). Establishment of practical recellularized liver graft for blood perfusion using primary rat hepatocytes and liver sinusoidal endothelial cells. American Journal of Transplantation, 18(6), 1351–1359
Mazza, G., Al-Akkad, W., Telese, A., Longato, L., Urbani, L., Robinson, B., et al. (2017). Rapid production of human liver scaffolds for functional tissue engineering by high shear stress oscillation-decellularization. Scientific Reports, 7(1), 5534
Banaeiyan, A. A., Theobald, J., Paukštyte, J., Wölfl, S., Adiels, C. B., & Goksör, M. (2017). Design and fabrication of a scalable liver-lobule-on-a-chip microphysiological platform. Biofabrication, 9(1), 015014
Yen, M. H., Wu, Y. Y., Liu, Y. S., Rimando, M., Ho, J. H., & Lee, O. K. (2016). Efficient generation of hepatic cells from mesenchymal stromal cells by an innovative bio-microfluidic cell culture device. Stem Cell Research & Therapy, 7(1), 120
Van der Helm, D., Groenewoud, A., de Jonge-Muller, E. S. M., Barnhoorn, M. C., Schoonderwoerd, M. J. A., Coenraad, M. J., et al. (2018). Mesenchymal stromal cells prevent progression of liver fibrosis in a novel zebrafish embryo model. Scientific Reports, 8(1), 16005
Tuñón, M. J., Alvarez, M., Culebras, J. M., & González-Gallego, J. (2009). An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World Journal of Gastroenterology, 15(25), 3086–3098
Yuan, L., Jiang, J., Liu, X., Zhang, Y., Zhang, L., Xin, J., et al. (2019). HBV infection-induced liver cirrhosis development in dual-humanised mice with human bone mesenchymal stem cell transplantation. Gut, 68(11), 2044–2056
Goessling, W., & Sadler, K. C. (2015). Zebrafish: an important tool for liver disease research. Gastroenterology, 149(6), 1361–1377
Jang, Y. O., Kim, Y. J., Baik, S. K., Kim, M. Y., Eom, Y. W., Cho, M. Y., et al. (2014). Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: a pilot study. Liver International, 34(1), 33–41
Andreone, P., Catani, L., Margini, C., Brodosi, L., Lorenzini, S., Sollazzo, D., et al. (2015). Reinfusion of highly purified CD133+ bone marrow-derived stem/progenitor cells in patients with end-stage liver disease: A phase I clinical trial. Digestive and Liver Disease, 47(12), 1059–1066
Zekri, A. R., Salama, H., Medhat, E., Musa, S., Abdel-Haleem, H., Ahmed, O. S., et al. (2015). The impact of repeated autologous infusion of haematopoietic stem cells in patients with liver insufficiency. Stem Cell Research & Therapy, 6(1), 118
Garg, V., Garg, H., Khan, A., Trehanpati, N., Kumar, A., Sharma, B. C., et al. (2012). Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology, 142(3), 505–5121
Mohamadnejad, M., Alimoghaddam, K., Bagheri, M., Ashrafi, M., Abdollahzadeh, L., Akhlaghpoor, S., et al. (2013). Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver International, 33(10), 1490–1496
Lanthier, N., Lin-Marq, N., Rubbia-Brandt, L., Clément, S., Goossens, N., & Spahr, L. (2017). Autologous bone marrow-derived cell transplantation in decompensated alcoholic liver disease: what is the impact on liver histology and gene expression patterns? Stem Cell Research & Therapy, 8(1), 88
Wang, S., Qu, X., & Zhao, R. C. (2012). Clinical applications of mesenchymal stem cells. Journal of Hematology & Oncology, 5, 19
Liang, J., Zhang, H., Zhao, C., Wang, D., Ma, X., Zhao, S., et al. (2017). Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases. International Journal of Rheumatic Diseases, 20(9), 1219–1226
Detry, O., Vandermeulen, M., Delbouille, M. H., Somja, J., Bletard, N., Briquet, A., et al. (2017). Infusion of mesenchymal stromal cells after deceased liver transplantation: A phase I-II, open-label, clinical study. Journal of Hepatology, 67(1), 47–55
Cernigliaro, V., Peluso, R., Zedda, B., Silengo, L., Tolosano, E., Pellicano, R., et al. (2020). Evolving cell-based and cell-free clinical strategies for treating severe human liver diseases. Cells, 9(2), 386
Sakiyama, R., Blau, B. J., & Miki, T. (2017). Clinical translation of bioartificial liver support systems with human pluripotent stem cell-derived hepatic cells. World Journal of Gastroenterology, 23(11), 1974–1979
Eom, Y. W., Yoon, Y., & Baik, S. K. (2021). Mesenchymal stem cell therapy for liver disease: current status and future perspectives. Current Opinion in Gastroenterology, 37(3), 216–223
Yang, X., Meng, Y., Han, Z., Ye, F., Wei, L., & Zong, C. (2020). Mesenchymal stem cell therapy for liver disease: full of chances and challenges. Cell & Bioscience, 10, 123
Funding
This work was supported by grants from the National Natural Science Foundation of China (11702044, 11902061), Natural Science Foundation of Chongqing (cstc2020jcyj-msxmX0350), Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN201901114), Visiting Scholar Foundation of Key Laboratory of Biorheological Science and Technology (Chongqing University), Ministry of Education (CQKLBST-2020-001), Scientific Research Foundation of Chongqing University of Technology (2019ZD49), and Scientific Research Foundation of Chongqing Technology and Business University (1956016).
Availability of Data and MaterialNot applicable.
Author information
Authors and Affiliations
Contributions
G. C. and Y. D. conceptualized the idea. Y. D., B. X., Z. C., F. W., Y. L. and G. C. wrote the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, Y., Xia, B., Chen, Z. et al. Stem Cell-based Therapy Strategy for Hepatic Fibrosis by Targeting Intrahepatic Cells. Stem Cell Rev and Rep 18, 77–93 (2022). https://doi.org/10.1007/s12015-021-10286-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10286-9