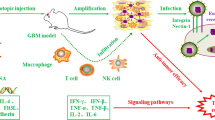
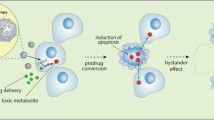
Abstract
Evasion of growth suppression is among the prominent hallmarks of cancer. Phosphatase and tensin homolog (PTEN) and p53 tumor-suppressive pathways are compromised in most human cancers, including glioblastoma (GB). Hence, these signaling pathways are an ideal point of focus for novel cancer therapeutics. Recombinant viruses can selectivity kill cancer cells and carry therapeutic genes to tumors. Specifically, oncolytic viruses (OV) have been successfully employed for gene delivery in GB animal models and showed potential to neutralize immunosuppression at the tumor site. However, the associated systemic immunogenicity, inefficient transduction of GB cells, and inadequate distribution to metastatic tumors have been the major bottlenecks in clinical studies. Mesenchymal stem cells (MSCs), with tumor-tropic properties and immune privilege, can improve OVs targeting. Remarkably, combining the two approaches can address their individual issues. Herein, we summarize findings to advocate the reactivation of tumor suppressors p53 and PTEN in GB treatment and use MSCs as a “Trojan horse” to carry oncolytic viral cargo to disseminated tumor beds. The integration of MSCs and OVs can emerge as the new paradigm in cancer treatment.
Graphical abstract





Similar content being viewed by others
Abbreviations
- Adv:
-
Adenovirus
- OA:
-
Oncolytic adenovirus
- OVs:
-
Oncolytic viruses
- PTEN:
-
Phosphatase and tensin homolog
- GB:
-
Glioblastoma
- GSC:
-
Glioma stem cell
- MSCs:
-
Mesenchymal stem cells
- PI3Ks:
-
Phosphatidylinositol 3-kinases
- AKT:
-
a serine/threonine-specific protein kinase
- PIP2 :
-
Phosphatidylinositol-4,5-bisphosphate
- CRAds:
-
Conditionally replicating adenoviruses
- RD-Ads:
-
Replication-defective adenoviral vectors
- TCGA:
-
The Cancer Genome Atlas
- MDM2:
-
Mouse double minute 2 homolog
- CDKN2A:
-
also known as cyclin-dependent kinase inhibitor 2A
- YB-1:
-
Y-box-binding protein-1
- ARF:
-
Alternative reading frame
- SOX2:
-
SRY-Box Transcription Factor 2
- FoxO1:
-
Forkhead Box O1
- SHH:
-
Sonic hedgehog
- PAX7:
-
Paired Box 7
- BRAF:
-
Serine/threonine-protein kinase B-raf
- B7-H1:
-
B7 homolog 1
- PD-L1:
-
Programmed death-ligand 1
- E2F1:
-
E2F Transcription Factor 1
- myc:
-
a proto-oncogene that code for transcription factors
- ras:
-
Rat sarcoma – a proto-oncogene
- ATM:
-
Ataxia Telangiectasia Mutated – a Serine/Threonine Kinase
- mTORC1:
-
Mammalian target of rapamycin complex 1
- GCV:
-
Ganciclovir
- HSV-TK:
-
Herpes Simplex Virus-1 Thymidine Kinase
- CD-NSCs:
-
Cytosine deaminase expressing neural stem cells
- optHBS:
-
Optimized HIF-binding site
- TRAIL:
-
tumor necrosis factor-related apoptosis-inducing ligand
- RNAi:
-
RNA interference
- RISC:
-
RNA-induced silencing complex
- siRNA/shRNA:
-
Small interfering and short hairpin RNA
- TME:
-
Tumor microenvironment
- IFN-β:
-
Interferon beta
- CAR:
-
Chimeric antigen receptor
- TCR-T:
-
Affinity-enhanced T cell receptor-transgenic T
- DOTMA:
-
N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethyl ammonium chloride
- DOTAP:
-
[1,2-bis (oleoyloxy)-3-(trimethylammonio) propane]
- DOPE:
-
Dioleoylphosphatidylethanolamine
- CXCR4:
-
C-X-C chemokine receptor type 4
- ICOVIR-5:
-
Oncolytic virus
- Celyvir:
-
Autologous mesenchymal stem cells carrying ICOVIR-5
- HER2:
-
Human epidermal growth factor receptor
References
Louis, D. N., et al. (2016). The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta neuropathologica, 131, 803–820.
Kim, Y. Z., et al. (2019). The Korean Society for Neuro-Oncology (KSNO) guideline for glioblastomas: version 2018.01. Brain tumor research and treatment 7, 1.
Cha, G. D., et al. (2020). Advances in drug delivery technology for the treatment of glioblastoma multiforme. Journal of Controlled Release, 328, 350–367.
Furnari, F. B., et al. (2007). Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes & development, 21, 2683–2710.
Arvanitis, C. D., Ferraro, G. B., & Jain, R. K. (2020). The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nature Reviews Cancer, 20, 26–41.
Lee, Y.-R., et al., (2019). Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science, 364, eaau0159.
Lee, E. Y. & Muller, W. J. (2010). Oncogenes and tumor suppressor genes. Cold Spring Harbor perspectives in biology. 2, a003236.
Cooper, G. M. & Hausman, R. E. The cell: Molecular approach. (Medicinska naklada, 2004).
Panek, W. K., et al. (2017). Hitting the nail on the head: Combining oncolytic adenovirus-mediated virotherapy and immunomodulation for the treatment of glioma. Oncotarget, 8, 89391.
Pikor, L. A., Bell, J. C., & Diallo, J.-S. (2015). Oncolytic viruses: Exploiting cancer’s deal with the devil. Trends in Cancer, 1, 266–277.
Namba, H., Kawaji, H., & Yamasaki, T. (2015). Use of genetically engineered stem cells for glioma therapy. Oncology letters, 11, 9–15.
Bexell, D., Svensson, A., & Bengzon, J. (2013). Stem cell-based therapy for malignant glioma. Cancer treatment reviews, 39, 358–365.
Brennan, C. (2011). Genomic profiles of glioma. Current neurology and neuroscience reports, 11, 291–297.
Tong, H., Zhao, K., Zhang, J., Zhu, J., & Xiao, J. (2019). YB-1 modulates the drug resistance of glioma cells by activation of MDM2/p53 pathway. Drug design, development and therapy, 13, 317.
Di Agostino, S., Fontemaggi, G., Strano, S., Blandino, G., & D’Orazi, G. (2019). Targeting mutant p53 in cancer: The latest insights. Journal of Experimental & Clinical Cancer Research, 38, 290.
Levine, A. J. (2019). Targeting therapies for the p53 protein in cancer treatments. Annual Review of Cancer Biology, 3, 21–34.
Pessôa, I. A., et al. (2019). Detection and Correlation of Single and Concomitant TP53, PTEN, and CDKN2A Alterations in Gliomas. International Journal of Molecular Sciences, 20, 2658.
Di Cristofano, A., et al. (1999). Impaired Fas response and autoimmunity in Pten+/− mice. Science, 285, 2122–2125.
Lawrence, M. S., et al. (2014). Discovery and saturation analysis of cancer genes across 21 tumour types. Nature, 505, 495–501.
Alimonti, A., et al. (2010). Subtle variations in Pten dose determine cancer susceptibility. Nature genetics, 42, 454–458.
Koul, D. (2008). PTEN signaling pathways in glioblastoma. Cancer biology & therapy, 7, 1321–1325.
Jiang, N., et al. (2020). Role of PI3K/AKT pathway in cancer: the framework of malignant behavior. Molecular Biology Reports, 1–43.
Lopez-Bertoni, H., et al. (2020). A Sox2: MiR-486-5p axis regulates survival of GBM cells by inhibiting tumor suppressor networks. Cancer research, 80, 1644–1655.
Gao, Z. G., Yang, P., Huang, J. & Ding, Y. Q. (2020). CircFBXW7 Alleviates Glioma Progression through Regulating MiR‐23a‐3p/PTEN Axis. The Anatomical Record.
Filbin, M. G., et al. (2013). Coordinate activation of Shh and PI3K signaling in PTEN-deficient glioblastoma: New therapeutic opportunities. Nature medicine, 19, 1518–1523.
Duan, S., et al. (2015). PTEN deficiency reprogrammes human neural stem cells towards a glioblastoma stem cell-like phenotype. Nature communications, 6, 1–14.
Ma, J., et al. (2019). Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell, 35, 504–518. e507.
Robinson, G. W., Orr, B. A., & Gajjar, A. (2014). Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer, 14, 1–5.
Parsa, A. T., et al. (2007). Loss of tumor suppressor PTEN function increases B7–H1 expression and immunoresistance in glioma. Nature medicine, 13, 84–88.
Zhao, J., et al. (2019). Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nature medicine, 25, 462–469.
Cetintas, V. B., & Batada, N. N. (2020). Is there a causal link between PTEN deficient tumors and immunosuppressive tumor microenvironment? Journal of Translational Medicine, 18, 45.
Rattanavirotkul, N., Kirschner, K. & Chandra, T. (2020). Induction and transmission of oncogene-induced senescence. Cellular and Molecular Life Sciences, 1–10.
Tonnessen-Murray, C. A., Lozano, G. & Jackson, J. G. The regulation of cellular functions by the p53 protein: cellular senescence. Cold Spring Harbor perspectives in medicine 7, a026112 (2017).
Bang, S., Kaur, S., & Kurokawa, M. (2020). Regulation of the p53 Family Proteins by the Ubiquitin Proteasomal Pathway. International Journal of Molecular Sciences, 21, 261.
Levine, A. J. (2020). p53: 800 million years of evolution and 40 years of discovery. Nature Reviews Cancer, 1–10.
Harris, S. L., & Levine, A. J. (2005). The p53 pathway: Positive and negative feedback loops. Oncogene, 24, 2899–2908.
Bond, G. L., Hu, W., & Levine, A. J. (2005). MDM2 is a central node in the p53 pathway: 12 years and counting. Current cancer drug targets, 5, 3–8.
Mendelsohn, J., Howley, P. M., Israel, M. A., Gray, J. W. & Thompson, C. B. (2008). The Molecular Basis of Cancer: Expert Consult-Online. (Elsevier Health Sciences).
Noorolyai, S., Shajari, N., Baghbani, E., Sadreddini, S., & Baradaran, B. (2019). The relation between PI3K/AKT signalling pathway and cancer. Gene, 698, 120–128.
Fruman, D. A., et al. (2017). The PI3K pathway in human disease. Cell, 170, 605–635.
de Olza, M. O., Rodrigo, B. N., Zimmermann, S., & Coukos, G. (2020). Turning up the heat on non-immunoreactive tumours: Opportunities for clinical development. The Lancet Oncology, 21, e419–e430.
Pons-Tostivint, E., Thibault, B., & Guillermet-Guibert, J. (2017). Targeting PI3K signaling in combination cancer therapy. Trends in cancer, 3, 454–469.
Wang, Y., Canine, B. F. & Hatefi, A. (2011). HSV-TK/GCV cancer suicide gene therapy by a designed recombinant multifunctional vector. Nanomedicine: Nanotechnology, Biology and Medicine, 7, 193–200.
Portnow, J., et al. (2017). Neural stem cell–based anticancer gene therapy: A first-in-human study in recurrent high-grade glioma patients. Clinical Cancer Research, 23, 2951–2960.
Huang, J., et al. (2010). Target gene therapy of glioma: Overexpression of BAX gene under the control of both tissue-specific promoter and hypoxia-inducible element. Acta Biochimica et Biophysica Sinica, 42, 274–280.
Perlstein, B., et al. (2013). TRAIL conjugated to nanoparticles exhibits increased anti-tumor activities in glioma cells and glioma stem cells in vitro and in vivo. Neuro-oncology, 15, 29–40.
Jiang, J., et al. (2014). A preliminary study on the construction of double suicide gene delivery vectors by mesenchymal stem cells and the in vitro inhibitory effects on SKOV3 cells. Oncology reports, 31, 781–787.
Okura, H., Smith, C. A., & Rutka, J. T. (2014). Gene therapy for malignant glioma. Molecular and cellular therapies, 2, 21.
Wang, K., Park, J. O., & Zhang, M. (2013). Treatment of glioblastoma multiforme using a combination of small interfering RNA targeting epidermal growth factor receptor and β-catenin. The journal of gene medicine, 15, 42–50.
Pottoo, F. H., Javed, M. N., Rahman, J. U., Abu-Izneid, T. & Khan, F. A. in Seminars in cancer biology. 391–398 (Elsevier).
Price, C., & Chen, J. (2014). MicroRNAs in cancer biology and therapy: Current status and perspectives. Genes & diseases, 1, 53–63.
Rezaei, T., et al. (2020). microRNA-181a mediates the chemo-sensitivity of glioblastoma to carmustine and regulates cell proliferation, migration, and apoptosis. European Journal of Pharmacology, 173483.
Geraldo, L. H. M., et al. (2019). Glioblastoma therapy in the age of molecular medicine. Trends in cancer, 5, 46–65.
Nakamura, K., et al. (2004). Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene therapy, 11, 1155–1164.
Nakamizo, A., et al. (2005). Human bone marrow–derived mesenchymal stem cells in the treatment of gliomas. Cancer research, 65, 3307–3318.
Mosallaei, M. et al. (2020). Genetically engineered mesenchymal stem cells: targeted delivery of immunomodulatory agents for tumor eradication. Cancer Gene Therapy, 1–15.
Rosenberg, S. A., & Restifo, N. P. (2015). Adoptive cell transfer as personalized immunotherapy for human cancer. Science, 348, 62–68.
Ecsedi, M., McAfee, M. S. & Chapuis, A. G. The Anticancer Potential of T Cell Receptor-Engineered T Cells. Trends in Cancer (2020).
Singh, A. K., & McGuirk, J. P. (2020). CAR T cells: Continuation in a revolution of immunotherapy. The Lancet Oncology, 21, e168–e178.
Spear, T. T., Evavold, B. D., Baker, B. M., & Nishimura, M. I. (2019). Understanding TCR affinity, antigen specificity, and cross-reactivity to improve TCR gene-modified T cells for cancer immunotherapy. Cancer Immunology, Immunotherapy, 68, 1881–1889.
O’Leary, M. C., et al. (2019). FDA approval summary: Tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clinical Cancer Research, 25, 1142–1146.
Hombach, A. A., Geumann, U., Günther, C., Hermann, F. G., & Abken, H. (2020). IL7-IL12 Engineered mesenchymal stem cells (MSCs) improve a CAR T cell attack against colorectal cancer cells. Cells, 9, 873.
Li, S. et al. (2020). Cancer immunotherapy via targeted TGF-β signalling blockade in TH cells. Nature, 1–5.
Corbeil, D. & Lorico, A. (2020). in Exosomes 39–80 (Elsevier).
Ohno, S.-I., et al. (2013). Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Molecular Therapy, 21, 185–191.
Shtam, T. A., et al. (2013). Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Communication and Signaling, 11, 88.
Kosaka, N., et al. (2010). Secretory mechanisms and intercellular transfer of microRNAs in living cells. Journal of Biological Chemistry, 285, 17442–17452.
Wang, K., Kievit, F. M., & Zhang, M. (2016). Nanoparticles for cancer gene therapy: Recent advances, challenges, and strategies. Pharmacological research, 114, 56–66.
Wang, A. Z., Langer, R., & Farokhzad, O. C. (2012). Nanoparticle delivery of cancer drugs. Annual review of medicine, 63, 185–198.
Simberg, D., Weisman, S., Talmon, Y. & Barenholz, Y. (2004). DOTAP (and other cationic lipids): chemistry, biophysics, and transfection. Critical Reviews™ in Therapeutic Drug Carrier Systems, 21.
Zhang, Y., et al. (2010). DC-Chol/DOPE cationic liposomes: A comparative study of the influence factors on plasmid pDNA and siRNA gene delivery. International journal of pharmaceutics, 390, 198–207.
Song, G., et al. (2014). Effects of tumor microenvironment heterogeneity on nanoparticle disposition and efficacy in breast cancer tumor models. Clinical Cancer Research, 20, 6083–6095.
Lewinski, N., Colvin, V., & Drezek, R. (2008). Cytotoxicity of nanoparticles. Small, 4, 26–49.
Ferraris, C., Cavalli, R., Panciani, P. P., & Battaglia, L. (2020). Overcoming the Blood-Brain Barrier: Successes and Challenges in Developing Nanoparticle-Mediated Drug Delivery Systems for the Treatment of Brain Tumours. International Journal of Nanomedicine, 15, 2999.
Drappatz, J., et al. (2013). Phase I study of GRN1005 in recurrent malignant glioma. Clinical Cancer Research, 19, 1567–1576.
Yeini, E., et al. (2021). Targeting Glioblastoma: Advances in Drug Delivery and Novel Therapeutic Approaches. Advanced Therapeutics, 4, 2000124.
Kiyokawa, J., & Wakimoto, H. (2019). Preclinical and clinical development of oncolytic adenovirus for the treatment of malignant glioma. Oncolytic virotherapy, 8, 27.
Srinivasan, V. M., Lang, F. F., & Kan, P. (2021). Intraarterial delivery of virotherapy for glioblastoma. Neurosurgical focus, 50, E7.
Sakhawat, A., et al. (2017). Upregulation of Coxsackie adenovirus receptor sensitizes cisplatin-resistant lung cancer cells to CRAd-induced inhibition. Journal of Cancer, 8, 1425.
Ali, S., et al. (2019). Cisplatin Synergistically Enhances Antitumor Potency of Conditionally Replicating Adenovirus via p53 Dependent or Independent Pathways in Human Lung Carcinoma. International Journal of Molecular Sciences, 20, 1125.
Sakhawat, A., et al. (2019). A tumor targeting oncolytic adenovirus can improve therapeutic outcomes in chemotherapy resistant metastatic human breast carcinoma. Scientific reports, 9, 1–11.
Eissa, I. R., et al. (2018). The current status and future prospects of oncolytic viruses in clinical trials against melanoma, glioma, pancreatic, and breast cancers. Cancers, 10, 356.
Conry, R. M., Westbrook, B., McKee, S., & Norwood, T. G. (2018). Talimogene laherparepvec: First in class oncolytic virotherapy. Human Vaccines & Immunotherapeutics, 14, 839–846. https://doi.org/10.1080/21645515.2017.1412896
Fan, X., et al. (2018). Overexpression of p53 delivered using recombinant NDV induces apoptosis in glioma cells by regulating the apoptotic signaling pathway. Experimental and therapeutic medicine, 15, 4522–4530.
Bressy, C., Hastie, E., & Grdzelishvili, V. Z. (2017). Combining oncolytic virotherapy with p53 tumor suppressor gene therapy. Molecular Therapy-Oncolytics, 5, 20–40.
Lang, F. F., et al. (2003). Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: Biological and clinical results. Journal of Clinical Oncology, 21, 2508–2518.
Devaraja, K. (2019). Current prospects of molecular therapeutics in head and neck squamous cell carcinoma. Pharmaceutical Medicine, 1–21.
Russell, L., et al. (2018). PTEN expression by an oncolytic herpesvirus directs T-cell mediated tumor clearance. Nature communications, 9, 1–16.
Nguyen, H.-M., & Saha, D. (2021). The Current State of Oncolytic Herpes Simplex Virus for Glioblastoma Treatment. Oncolytic Virotherapy, 10, 1.
Bommareddy, P. K., Shettigar, M., & Kaufman, H. L. (2018). Integrating oncolytic viruses in combination cancer immunotherapy. Nature Reviews Immunology, 18, 498.
Twumasi-Boateng, K., Pettigrew, J. L., Kwok, Y. E., Bell, J. C., & Nelson, B. H. (2018). Oncolytic viruses as engineering platforms for combination immunotherapy. Nature Reviews Cancer, 18, 419–432.
Lang, F. F., et al. (2018). Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: Replication and immunotherapeutic effects in recurrent malignant glioma. Journal of Clinical Oncology, 36, 1419.
Belcaid, Z. et al. (2020). Low-dose oncolytic adenovirus therapy overcomes tumor-induced immune suppression and sensitizes intracranial gliomas to anti-PD-1 therapy. Neuro-Oncology Advances, 2, vdaa011.
Ribas, A., et al. (2017). Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 170, 1109–1119. e1110.
Ulasov, I. V., et al. (2009). Oncolytic adenoviral vectors which employ the survivin promoter induce glioma oncolysis via a process of beclin-dependent autophagy. International journal of oncology, 34, 729–742.
Trus, I., et al. (2020). Zika Virus with Increased CpG Dinucleotide Frequencies Shows Oncolytic Activity in Glioblastoma Stem Cells. Viruses, 12, 579.
Goradel, N. H., Negahdari, B., Ghorghanlu, S., Jahangiri, S. & Arashkia, A. (2020). Strategies for enhancing intratumoral spread of oncolytic adenoviruses. Pharmacology & Therapeutics, 107586.
Pisklakova, A., et al. (2016). M011L-deficient oncolytic myxoma virus induces apoptosis in brain tumor-initiating cells and enhances survival in a novel immunocompetent mouse model of glioblastoma. Neuro-oncology, 18, 1088–1098.
Eissa, I. R. et al. Oncolytic herpes simplex virus HF10 (canerpaturev, C-REV) promotes accumulation of CD8+ PD-1-tumor-infiltrating T cells in PD-L1-enriched tumor microenvironment. International journal of cancer.
Tang, B., et al. (2020). Synergistic Combination of Oncolytic Virotherapy and Immunotherapy for Glioma. Clinical Cancer Research, 26, 2216–2230.
Eriksson, E., et al. (2017). Shaping the tumor stroma and sparking immune activation by CD40 and 4–1BB signaling induced by an armed oncolytic virus. Clinical Cancer Research, 23, 5846–5857.
Buchan, S. L., Rogel, A., & Al-Shamkhani, A. (2018). The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood, The Journal of the American Society of Hematology, 131, 39–48.
Liu, X. R., et al. (2012). A new oncolytic adenoviral vector carrying dual tumour suppressor genes shows potent anti-tumour effect. Journal of cellular and molecular medicine, 16, 1298–1309.
Chen, P., et al. (2019). The construction and characterization of a novel adenovirus vector of artificial microRNA targeting EGFR. International Journal of Clinical and Experimental Pathology, 12, 1968.
Zhang, Y., & Liu, Z. (2019). Oncolytic Virotherapy for Malignant Tumor: Current Clinical Status. Current pharmaceutical design, 25, 4251–4263.
Hall, B., Andreeff, M. & Marini, F. (2007). in Bone Marrow-Derived Progenitors 263–283 (Springer).
Studeny, M., et al. (2002). Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumors. Cancer research, 62, 3603–3608.
Son, B. R., et al. (2006). Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem cells, 24, 1254–1264.
Shen, C.-J., et al. (2016). Human umbilical cord matrix-derived stem cells expressing interferon-β gene inhibit breast cancer cells via apoptosis. Oncotarget, 7, 34172.
Liu, X., et al. (2018). Mesenchymal stem cells expressing interleukin-18 inhibit breast cancer in a mouse model. Oncology letters, 15, 6265–6274.
Mangraviti, A., et al. (2016). Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials, 100, 53–66.
Guo, X.-R., et al. (2016). The application of mRNA-based gene transfer in mesenchymal stem cell-mediated cytotoxicity of glioma cells. Oncotarget, 7, 55529.
Lang, F. M., et al. (2018). Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro-oncology, 20, 380–390.
Li, X., Wang, K. & Ai, H. (2019). Human umbilical cord mesenchymal stem cell-derived extracellular vesicles inhibit endometrial cancer cell proliferation and migration through delivery of exogenous miR-302a. Stem Cells International, 2019.
Pacioni, S., et al. (2015). Mesenchymal stromal cells loaded with paclitaxel induce cytotoxic damage in glioblastoma brain xenografts. Stem cell research & therapy, 6, 1–11.
Sharif, S., Ghahremani, M. H. & Soleimani, M. Differentiation Induction and Proliferation Inhibition by A Cell-Free Approach for Delivery of Exogenous miRNAs to Neuroblastoma Cells Using Mesenchymal Stem Cells. Cell journal 22, 556.
Li, M., et al. (2019). Exploiting tumor-intrinsic signals to induce mesenchymal stem cell-mediated suicide gene therapy to fight malignant glioma. Stem cell research & therapy, 10, 1–15.
Mohme, M., et al. (2020). Local intracerebral immunomodulation using interleukin-expressing mesenchymal stem cells in glioblastoma. Clinical Cancer Research, 26, 2626–2639.
Fan, S. et al. Umbilical cord-derived mesenchymal stromal/stem cells expressing IL-24 induce apoptosis in gliomas. 235, 1769–1779, doi:https://doi.org/10.1002/jcp.29095 (2020).
Duebgen, M. et al. Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. JNCI: Journal of the National Cancer Institute 106 (2014).
Leoni, V., et al. (2015). Systemic delivery of HER2-retargeted oncolytic-HSV by mesenchymal stromal cells protects from lung and brain metastases. Oncotarget, 6, 34774.
Thaci, B., et al. (2012). Pharmacokinetic study of neural stem cell-based cell carrier for oncolytic virotherapy: Targeted delivery of the therapeutic payload in an orthotopic brain tumor model. Cancer gene therapy, 19, 431–442.
Jobst, C., et al. (2017). Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells-TREAT-ME-1-a phase I, first in human, first in class trial. Oncotarget, 8, 80156.
Hadryś, A., Sochanik, A., McFadden, G. & Jazowiecka-Rakus, J. (2020). Mesenchymal stem cells as carriers for systemic delivery of oncolytic viruses. European Journal of Pharmacology, 172991.
Karnoub, A. E., et al. (2007). Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature, 449, 557–563.
Klopp, A. H. et al. (2010). Mesenchymal stem cells promote mammosphere formation and decrease E-cadherin in normal and malignant breast cells. PloS one, 5, e12180.
Qiao, L., Xu, Z.-L., Zhao, T.-J., Ye, L.-H., & Zhang, X.-D. (2008). Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer letters, 269, 67–77.
Sasportas, L. S., et al. (2009). Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proceedings of the national academy of sciences, 106, 4822–4827.
Behnan, J., et al. (2014). Recruited brain tumor-derived mesenchymal stem cells contribute to brain tumor progression. Stem Cells, 32, 1110–1123.
Pulkkanen, K. J., & Yla-Herttuala, S. (2005). Gene therapy for malignant glioma: Current clinical status. Molecular Therapy, 12, 585–598.
Alvarez-Breckenridge, C. A., et al. (2012). NK cells impede glioblastoma virotherapy through NKp30 and NKp46 natural cytotoxicity receptors. Nature medicine, 18, 1827–1834.
Luo, Y., et al. (2020). Tumor-targeting oncolytic virus elicits potent immunotherapeutic vaccine responses to tumor antigens. Oncoimmunology, 9, 1726168.
Galland, S., & Stamenkovic, I. (2020). Mesenchymal stromal cells in cancer: A review of their immunomodulatory functions and dual effects on tumor progression. The Journal of pathology, 250, 555–572.
Aboody, K., Najbauer, J., & Danks, M. (2008). Stem and progenitor cell-mediated tumor selective gene therapy. Gene therapy, 15, 739–752.
Sonabend, A. M., et al. (2008). Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem cells, 26, 831–841.
Martinez-Quintanilla, J., He, D., Wakimoto, H., Alemany, R., & Shah, K. (2015). Encapsulated stem cells loaded with hyaluronidase-expressing oncolytic virus for brain tumor therapy. Molecular therapy, 23, 108–118.
Yong, R. L., et al. (2009). Human bone marrow–derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Δ24-RGD to human gliomas. Cancer research, 69, 8932–8940.
Hsiao, W.-C., Sung, S.-Y., Liao, C.-H., Wu, H.-C., & Hsieh, C.-L. (2012). Vitamin D3-inducible mesenchymal stem cell-based delivery of conditionally replicating adenoviruses effectively targets renal cell carcinoma and inhibits tumor growth. Molecular pharmaceutics, 9, 1396–1408.
Fei, S., et al. (2012). The antitumor effect of mesenchymal stem cells transduced with a lentiviral vector expressing cytosine deaminase in a rat glioma model. Journal of cancer research and clinical oncology, 138, 347–357.
Dührsen, L., et al. (2019). Preclinical analysis of human mesenchymal stem cells: Tumor tropism and therapeutic efficiency of local HSV-TK suicide gene therapy in glioblastoma. Oncotarget, 10, 6049.
Mahasa, K. J., et al. (2020). Mesenchymal stem cells used as carrier cells of oncolytic adenovirus results in enhanced oncolytic virotherapy. Scientific reports, 10, 1–13.
Prager, B. C., Bhargava, S., Mahadev, V., Hubert, C. G., & Rich, J. N. (2020). Glioblastoma Stem Cells: Driving Resilience through Chaos. Trends in Cancer, 6, 223–235.
Kazimirsky, G., Jiang, W., Slavin, S., Ziv-Av, A., & Brodie, C. (2016). Mesenchymal stem cells enhance the oncolytic effect of Newcastle disease virus in glioma cells and glioma stem cells via the secretion of TRAIL. Stem cell research & therapy, 7, 149.
Ruano, D., et al. (2020). First-in-human, first-in-child trial of autologous MSCs carrying the oncolytic virus Icovir-5 in patients with advanced tumors. Molecular Therapy.
Franco-Luzón, L., et al. (2020). Systemic oncolytic adenovirus delivered in mesenchymal carrier cells modulate tumor infiltrating immune cells and tumor microenvironment in mice with neuroblastoma. Oncotarget, 11, 347.
Na, Y., et al. (2019). Systemic administration of human mesenchymal stromal cells infected with polymer-coated oncolytic adenovirus induces efficient pancreatic tumor homing and infiltration. Journal of Controlled Release, 305, 75–88.
Ankrum, J. A., Ong, J. F., & Karp, J. M. (2014). Mesenchymal stem cells: Immune evasive, not immune privileged. Nature biotechnology, 32, 252.
Franquesa, M., Hoogduijn, M. J., Bestard, O., & Grinyó, J. M. (2012). Immunomodulatory effect of mesenchymal stem cells on B cells. Frontiers in immunology, 3, 212.
Najar, M., et al. (2016). Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy, 18, 160–171.
Mamidi, M. K., et al. (2012). Comparative cellular and molecular analyses of pooled bone marrow multipotent mesenchymal stromal cells during continuous passaging and after successive cryopreservation. Journal of cellular biochemistry, 113, 3153–3164.
Gao, P., Ding, Q., Wu, Z., Jiang, H., & Fang, Z. (2010). Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer letters, 290, 157–166.
Rincón, E., et al. (2017). Mesenchymal stem cell carriers enhance antitumor efficacy of oncolytic adenoviruses in an immunocompetent mouse model. Oncotarget, 8, 45415.
Guo, Y., et al. (2019). Menstrual blood-derived stem cells as delivery vehicles for oncolytic adenovirus virotherapy for colorectal cancer. Stem cells and development, 28, 882–896.
Du, W., et al. (2017). Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proceedings of the National Academy of Sciences, 114, E6157–E6165.
Muhammad, T., et al. (2019). Mesenchymal stem cell-mediated delivery of therapeutic adenoviral vectors to prostate cancer. Stem cell research & therapy, 10, 190.
Schenk, E., et al. (2014). Preclinical safety assessment of Ad [I/PPT-E1A], a novel oncolytic adenovirus for prostate cancer. Human Gene Therapy Clinical Development, 25, 7–15.
Robilotti, E. V., Kumar, A., Glickman, M. S., & Kamboj, M. (2019). Viral oncolytic immunotherapy in the war on cancer: Infection control considerations. Infection Control & Hospital Epidemiology, 40, 350–354.
Russell, S. J., Peng, K.-W., & Bell, J. C. (2012). Oncolytic virotherapy. Nature Biotechnology, 30, 658–670. https://doi.org/10.1038/nbt.2287
von Einem, J. C., et al. (2019). Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: Results from the phase 1/2 TREAT-ME-1 trial. International Journal of Cancer, 145, 1538–1546. https://doi.org/10.1002/ijc.32230
Shah, K. (2012). Mesenchymal stem cells engineered for cancer therapy. Advanced drug delivery reviews, 64, 739–748.
Kalimuthu, S., et al. (2018). A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Frontiers in pharmacology, 9, 1116.
Johansson, M., et al. (2013). The soluble form of the tumor suppressor Lrig1 potently inhibits in vivo glioma growth irrespective of EGF receptor status. Neuro-oncology, 15, 1200–1211.
Rossignoli, F., et al. (2019). Inducible Caspase9-mediated suicide gene for MSC-based cancer gene therapy. Cancer Gene Therapy, 26, 11–16. https://doi.org/10.1038/s41417-018-0034-1
Ramos, C. A., et al. (2010). An inducible caspase 9 suicide gene to improve the safety of mesenchymal stromal cell therapies. Stem Cells, 28, 1107–1115. https://doi.org/10.1002/stem.433
Neftel, C., et al. (2019). An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell, 178, 835–849. e821.
Mastrolia, I., et al. (2019). Challenges in Clinical Development of Mesenchymal Stromal/Stem Cells: Concise Review. Stem cells translational medicine, 8, 1135–1148. https://doi.org/10.1002/sctm.19-0044
De Becker, A., & Riet, I. V. (2016). Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World journal of stem cells, 8, 73–87. https://doi.org/10.4252/wjsc.v8.i3.73
Kean, T. J., Lin, P., Caplan, A. I., & Dennis, J. E. (2013). MSCs: Delivery Routes and Engraftment, Cell-Targeting Strategies, and Immune Modulation. Stem Cells Int, 2013, 732742. https://doi.org/10.1155/2013/732742
Shah, K. (2013). Encapsulated stem cells for cancer therapy. Biomatter, 3. https://doi.org/10.4161/biom.24278.
Smith, C. L., et al. (2015). Pre-exposure of human adipose mesenchymal stem cells to soluble factors enhances their homing to brain cancer. Stem cells translational medicine, 4, 239–251. https://doi.org/10.5966/sctm.2014-0149
Egea, V., et al. (2011). TNF-α respecifies human mesenchymal stem cells to a neural fate and promotes migration toward experimental glioma. Cell death and differentiation, 18, 853–863. https://doi.org/10.1038/cdd.2010.154
Kim, S. M., et al. (2011). CXC chemokine receptor 1 enhances the ability of human umbilical cord blood-derived mesenchymal stem cells to migrate toward gliomas. Biochemical and biophysical research communications, 407, 741–746. https://doi.org/10.1016/j.bbrc.2011.03.093
Balyasnikova, I. V., Franco-Gou, R., Mathis, J. M., & Lesniak, M. S. (2010). Genetic modification of mesenchymal stem cells to express a single-chain antibody against EGFRvIII on the cell surface. Journal of tissue engineering and regenerative medicine, 4, 247–258. https://doi.org/10.1002/term.228
Zhu, Y., et al. (2017). Bi-specific molecule against EGFR and death receptors simultaneously targets proliferation and death pathways in tumors. Science and Reports, 7, 2602. https://doi.org/10.1038/s41598-017-02483-9
Serakinci, N., & Cagsin, H. (2019). Programming hMSCs into Potential Genetic Therapy in Cancer. Critical reviews in eukaryotic gene expression, 29, 343–350. https://doi.org/10.1615/CritRevEukaryotGeneExpr.2019030483
Erenpreisa, J., Salmina, K., Anatskaya, O. & Cragg, M. S. in Seminars in Cancer Biology. (Elsevier).
Guterres, A. N., & Villanueva, J. (2020). Targeting telomerase for cancer therapy. Oncogene, 39, 5811–5824.
Friedman, G. K., et al. (2018). Enhanced sensitivity of patient-derived pediatric high-grade brain tumor xenografts to oncolytic HSV-1 virotherapy correlates with nectin-1 expression. Scientific reports, 8, 1–10.
Lee, Y.-R., et al. (2019). Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science, 364.
Liu, M., et al. (2020). TGF-β suppresses type 2 immunity to cancer. Nature, 587, 115–120. https://doi.org/10.1038/s41586-020-2836-1
Groeneveldt, C., van Hall, T., van der Burg, S. H., Ten Dijke, P. & van Montfoort, N. (2020). Immunotherapeutic Potential of TGF-β Inhibition and Oncolytic Viruses. Trends in Immunology.
Saha, D., Martuza, R. L. & Rabkin, S. D. (2017). Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer cell, 32, 253–267. e255.
Acknowledgements
The authors gratefully acknowledge the support from the Beijing Natural Science Foundation (Z190018), the National Natural Science Foundation of China (81870123), National Science Foundation for Young Scientists of China (81902545), and China Postdoctoral Science Foundation Grant (2018M641206). Figures were drawn using BioRender.com
Author information
Authors and Affiliations
Contributions
All authors took part in the preparation of this manuscript. SA and LD were responsible for determining the topic and idea. XQ and AAK drew figures/tables with the help of SA and performed literature search. XQ, YH, MT and DBC contributed with SA in writing the first draft and furtherly editing/revising the manuscript. LD, LL and XM perform extensive literature search and help to revise the manuscript in the light of queries and suggestions of the reviewers. All authors approved the version of the manuscript published. Overall supervision was done by LD.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethics approval was required for this review that did not involve patients or patient data.
Consent for publication
All authors consent to publication.
Competing interests
The authors declare that they have no competing interests.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, S., Xia, Q., Muhammad, T. et al. Glioblastoma Therapy: Rationale for a Mesenchymal Stem Cell-based Vehicle to Carry Recombinant Viruses. Stem Cell Rev and Rep 18, 523–543 (2022). https://doi.org/10.1007/s12015-021-10207-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10207-w