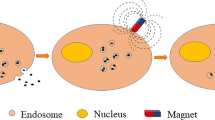
Abstract
Magnetic targeting (MT) has been an emerging technology which is used to improve the delivery and retention of transplanted therapeutic cells in target site over the past 20 years. Meanwhile, stem cells have also been a research hotspot in cell therapy in recent years. Several researchers have combined the MT technology with Stem cell therapy in order to improve the efficacy. However, Different types of Magnetic Nano particles (MNPs) have presented different effects, and how to choose a proper MNPs became a question. This article aims to introduce the preparation method and application field of different types of magnetic Nanoparticles, discuss the pros and cons of different types of MNPs in stem cell therapy and make a prospect of MT technology in Stem cell therapy.






Similar content being viewed by others
References
Beeres, S. L., Bengel, F. M., Bartunek, J., Atsma, D. E., Hill, J. M., Vanderheyden, M., Penicka, M., Schalij, M. J., Wijns, W., & Bax, J. J. (2007). Role of imaging in cardiac stem cell therapy. Journal of the American College of Cardiology, 49, 1137–1148.
Adams, C. F., Rai, A., Sneddon, G., Yiu, H. H., Polyak, B., & Chari, D. M. (2015). Increasing magnetite contents of polymeric magnetic particles dramatically improves labeling of neural stem cell transplant populations. Nanomedicine, 11, 19–29. https://doi.org/10.1016/j.nano.2014.07.001.
Shen, W. B., Plachez, C., Tsymbalyuk, O., Tsymbalyuk, N., Xu, S., Smith, A. M., & Yarowsky, P. (2016). Cell-based therapy in TBI: Magnetic retention of neural stem cells in vivo. Cell. Transplantation, 25, 1085–1099.
Ramos-Gomez, M., & Martinez-Serrano, A. (2016). Tracking of iron-labeled human neural stem cells by magnetic resonance imaging in cell replacement therapy for Parkinson's disease. Neural Regeneration Research, 11, 49–52.
Blurton-Jones, M., Kitazawa, M., Martinez-Coria, H., Castello, N. A., Müller, F.-J., Loring, J. F., et al. (2009). Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proceedings of the National Academy of Sciences, 106, 13594–13599.
Alipour, M., Nabavi, S. M., Arab, L., Vosough, M., Pakdaman, H., Ehsani, E., & Shahpasand, K. (2019). Stem cell therapy in Alzheimer's disease: Possible benefits and limiting drawbacks. Molecular Biology Reports, 46, 1425–1446.
Deng, B., Wen, J., Ding, Y., Peng, J., & Jiang, S. (2012). Different regulation role of myostatin in differentiating pig ADSCs and MSCs into adipocytes. Cell Biochemistry and Function, 30, 145–150.
Tukmachev, D., Lunov, O., Zablotskii, V., Dejneka, A., Babic, M., Sykova, E., & Kubinova, S. (2015). An effective strategy of magnetic stem cell delivery for spinal cord injury therapy. Nanoscale, 7, 3954–3958.
Senyei, A., Widder, K., & Czerlinski, G. (1978). Magnetic guidance of drug-carrying microspheres. Journal of Applied Physics, 49, 3578–3583.
Widder, K. J., Senyei, A. E., & Scarpelli, D. G. (1978). Magnetic microspheres: A model system for site specific drug delivery in vivo. Proceedings of the Society for Experimental Biology and Medicine, 158, 141–146.
McBain, S. C., Yiu, H. H., & Dobson, J. (2008). Magnetic nanoparticles for gene and drug delivery. International Journal of Nanomedicine,3, 169–180.
Cores, J., Caranasos, T., & Cheng, K. (2015). Magnetically targeted stem cell delivery for regenerative medicine. Journal of Functional Biomaterials, 6, 526–546.
Cheng, K., Shen, D., Hensley, M. T., Middleton, R., Sun, B., Liu, W., et al. (2014). Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nature Communications, 5, 4880. https://doi.org/10.1038/ncomms5880.
Arbab, A. S., Jordan, E. K., Wilson, L. B., Yocum, G. T., Lewis, B. K., & Frank, J. A. (2004). In vivo trafficking and targeted delivery of magnetically labeled stem cells. Human Gene Therapy, 15, 351–360.
Rudelius, M., Daldrup-Link, H. E., Heinzmann, U., Piontek, G., Settles, M., Link, T. M., & Schlegel, J. (2003). Highly efficient paramagnetic labelling of embryonic and neuronal stem cells. European journal of nuclear and medicine molecular imaging, 30, 1038–1044.
Taylor, A., Herrmann, A., Moss, D., See, V., Davies, K., Williams, S. R., & Murray, P. (2014). Assessing the efficacy of nano- and micro-sized magnetic particles as contrast agents for MRI cell tracking. PLoS One, 9, e100259. https://doi.org/10.1371/journal.pone.0100259.
Rotherham, M., & El Haj, A. J. (2015). Remote activation of the Wnt/beta-catenin signalling pathway using functionalised magnetic particles. PLoS One, 10, e0121761. https://doi.org/10.1371/journal.pone.0121761.
Rahmi, G., Pidial, L., Silva, A. K. A., Blondiaux, E., Meresse, B., Gazeau, F., et al. (2016). Designing 3D Mesenchymal stem cell sheets merging magnetic and fluorescent features: When cell sheet technology meets image-guided cell therapy. Theranostics, 6, 739–751.
Suh, J. S., Lee, J. Y., Choi, Y. S., Yu, F., Yang, V., Lee, S. J., Chung, C. P., & Park, Y. J. (2009). Efficient labeling of mesenchymal stem cells using cell permeable magnetic nanoparticles. Biochemical biophysical research communications, 379, 669–675.
Ruan, J., Ji, J., Song, H., Qian, Q., Wang, K., Wang, C., & Cui, D. (2012). Fluorescent magnetic nanoparticle-labeled mesenchymal stem cells for targeted imaging and hyperthermia therapy of in vivo gastric cancer. Nanoscale Research Letters, 7(1), 309.
Cheng, K., Li, T. S., Malliaras, K., Davis, D. R., Zhang, Y., & Marban, E. (2010). Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circulation Research, 106, 1570–1581. https://doi.org/10.1161/CIRCRESAHA.109.212589.
Cheng, K., K. Malliaras, T.-S. Li, B. Sun, C. Houde, G. Galang, . . . E. Marbán. (2012). Magnetic enhancement of cell retention, engraftment, and functional benefit after intracoronary delivery of cardiac-derived stem cells in a rat model of ischemia/reperfusion. Cell Transplantation.
Fagg, W. S., Liu, N., Yang, M. J., Cheng, K., Chung, E., Kim, J. S., Wu, G., & Fair, J. (2017). Magnetic targeting of stem cell derivatives enhances hepatic engraftment into structurally Normal liver. Cell Transplantation, 26, 1868–1877. https://doi.org/10.1177/0963689717737320.
Vandergriff, A. C., Hensley, T. M., Henry, E. T., Shen, D., Anthony, S., Zhang, J., & Cheng, K. (2014). Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials, 35, 8528–8539. https://doi.org/10.1016/j.biomaterials.2014.06.031.
Lu, C.-W., Hung, Y., Hsiao, J.-K., Yao, M., Chung, T.-H., Lin, Y.-S., et al. (2007). Bifunctional magnetic silica nanoparticles for highly efficient human stem cell labeling. Nano Letters, 7, 149–154.
Ren, C., Li, J., Chen, X., Hu, Z., & Xue, D. (2007). Preparation and properties of a new multifunctional material composed of superparamagnetic core and rhodamine B doped silica shell. Nanotechnology, 18, 345604.
Nguyen, D. T., Tran, T. B., Nguyen, P. D., & Min, J. (2016). Addressing of LnCaP cell using magnetic particles assisted Impedimetric microelectrode. Journal of Nanoscience and Nanotechnology, 16, 2933–2936.
Chen, P. J., Kang, Y. D., Lin, C. H., Chen, S. Y., Hsieh, C. H., Chen, Y. Y., et al. (2015). Multitheragnostic multi-GNRs crystal-seeded magnetic Nanoseaurchin for enhanced in vivo Mesenchymal-stem-cell homing, multimodal imaging, and stroke therapy. Advanced Materials, 27(41), 6488–6495.
Marty, J. (1978). Nanoparticles-a new colloidal drug delivery system. Pharmaceutica Acta Helvetiae, 53, 17–23.
Xie, L., Tong, W., Yu, D., Xu, J., Li, J., & Gao, C. (2012). Bovine serum albumin nanoparticles modified with multilayers and aptamers for pH-responsive and targeted anti-cancer drug delivery. Journal of Materials Chemistry, 22, 6053–6060.
Han, J., Wang, Q., Zhang, Z., Gong, T., & Sun, X. (2014). Cationic bovine serum albumin based self-assembled nanoparticles as siRNA delivery vector for treating lung metastatic cancer. Small, 10, 524–535.
Li, F., Yang, G., Aguilar, Z. P., Xiong, Y., & Xu, H. (2018). Affordable and simple method for separating and detecting ovarian cancer circulating tumor cells using BSA coated magnetic nanoprobes modified with folic acid. Sensors Actuators B: Chemical, 262, 611–618.
Jiang, P., Zhang, Y., Zhu, C., Zhang, W., Mao, Z., & Gao, C. (2016). Fe3O4/BSA particles induce osteogenic differentiation of mesenchymal stem cells under static magnetic field. Acta Biomaterialia, 46, 141–150. https://doi.org/10.1016/j.actbio.2016.09.020.
Babic, M., Horák, D., Trchová, M., Jendelová, P., Glogarová, K., Lesný, P., et al. (2008). Poly (L-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjugate Chemistry, 19, 740–750.
Siow, W. X., Chang, Y.-T., Babič, M., Lu, Y.-C., Horák, D., & Ma, Y.-H. (2018). Interaction of poly-L-lysine coating and heparan sulfate proteoglycan on magnetic nanoparticle uptake by tumor cells. International Journal of Nanomedicine, 13, 1693.
Riggio, C., Calatayud, M. P., Hoskins, C., Pinkernelle, J., Sanz, B., Torres, T. E., et al. (2012). Poly-l-lysine-coated magnetic nanoparticles as intracellular actuators for neural guidance. International Journal of Nanomedicine, 7, 3155.
Kim, T. H., Kim, J. K., Shim, W., Kim, S. Y., Park, T. J., & Jung, J. Y. (2010). Tracking of transplanted mesenchymal stem cells labeled with fluorescent magnetic nanoparticle in liver cirrhosis rat model with 3-T MRI. Magnetic Resonance Imaging, 28, 1004–1013. https://doi.org/10.1016/j.mri.2010.03.047.
Frank, J. A., Miller, B. R., Arbab, A. S., Zywicke, H. A., Jordan, E. K., Lewis, B. K., Bryant LH Jr, & Bulte, J. W. (2003). Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology, 228, 480–487. https://doi.org/10.1148/radiol.2281020638.
Arbab, A. S., Bashaw, L. A., Miller, B. R., Jordan, E. K., Lewis, B. K., Kalish, H., & Frank, J. A. (2003). Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology, 229, 838–846. https://doi.org/10.1148/radiol.2293021215.
Ravikumar, C., Kumar, S., & Bandyopadhyaya, R. (2012). Aggregation of dextran coated magnetic nanoparticles in aqueous medium: Experiments and Monte Carlo simulation. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 403, 1–6. https://doi.org/10.1016/j.colsurfa.2012.02.007.
Hong, R., Feng, B., Chen, L., Liu, G., Li, H., Zheng, Y., & Wei, D. (2008). Synthesis, characterization and MRI application of dextran-coated Fe3O4 magnetic nanoparticles. Biochemical Engineering Journal, 42, 290–300.
Khalkhali, M., Sadighian, S., Rostamizadeh, K., Khoeini, F., Naghibi, M., Bayat, N., Habibizadeh, M., & Hamidi, M. (2015). Synthesis and characterization of dextran coated magnetite nanoparticles for diagnostics and therapy. Bioimpacts, 5, 141–150. https://doi.org/10.15171/bi.2015.19.
Soares, I. P., Ferreira, P. I. M. M., Igreja, R. A. G. B. N., Novo, C. M. M., & Borges, J. P. M. R. (2012). Application of hyperthermia for cancer treatment: Recent patents review. Recent Patents on Anti-Cancer Drug Discovery, 7, 64–73.
Jordan, A., Scholz, R., Wust, P., Schirra, H., Schiestel, T., Schmidt, H., & Felix, R. (1999). Endocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitro. Journal of Magnetism and Magnetic Materials, 194, 185–196.
Subramani, K. (2006). Applications of nanotechnology in drug delivery systems for the treatment of cancer and diabetes. International Journal of Nanotechnology, 3, 557–580.
Illés, E., Tombácz, E., Szekeres, M., Tóth, I. Y., Szabó, Á., & Iván, B. (2015). Novel carboxylated PEG-coating on magnetite nanoparticles designed for biomedical applications. Journal of Magnetism and Magnetic Materials, 380, 132–139.
Cole, A. J., David, A. E., Wang, J., Galban, C. J., & Yang, V. C. (2011). Magnetic brain tumor targeting and biodistribution of long-circulating PEG-modified, cross-linked starch-coated iron oxide nanoparticles. Biomaterials, 32, 6291–6301. https://doi.org/10.1016/j.biomaterials.2011.05.024.
Landázuri, N., S. Tong, J. Suo, G. Joseph, D. Weiss, D.J. Sutcliffe, D.P. Giddens, G. Bao W.R. Taylor. (2013). Magnetic targeting of human mesenchymal stem cells with internalized superparamagnetic iron oxide nanoparticles. Small, 9, 4017–4026.
Bu, L. L., Rao, L., Yu, G. T., Chen, L., Deng, W. W., Liu, J. F., et al. (2019). Cancer stem cell-platelet hybrid membrane-coated magnetic nanoparticles for enhanced Photothermal therapy of head and neck squamous cell carcinoma. Advanced Functional Materials, 29, 1807733.
Alexiou, C., Arnold, W., Hulin, P., Klein, R. J., Renz, H., Parak, F. G., et al. (2001). Magnetic mitoxantrone nanoparticle detection by histology, X-ray and MRI after magnetic tumor targeting. Journal of Magnetism and Magnetic Materials, 225, 187–193.
Cheraghipour, E., Javadpour, S., & Mehdizadeh, A. R. (2012). Citrate capped superparamagnetic iron oxide nanoparticles used for hyperthermia therapy. Journal of Biomedical Science and Engineering, 5, 715.
Connell, J. J., Patrick, P. S., Yu, Y., Lythgoe, M. F., & Kalber, T. L. (2015). Advanced cell therapies: Targeting, tracking and actuation of cells with magnetic particles. Regenerative Medicine, 10, 757–772. https://doi.org/10.2217/rme.15.36.
Lewinski, N., Colvin, V., & Drezek, R. (2008). Cytotoxicity of nanoparticles. Small, 4, 26–49. https://doi.org/10.1002/smll.200700595.
Voinov, M. A., Pagán, J. O. S., Morrison, E., Smirnova, T. I., & Smirnov, A. I. (2010). Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. Journal of the American Chemical Society, 133, 35–41.
Blomberg, B., Geckeler, W. R., & Weigert, M. (1972). Genetics of the antibody response to dextran in mice. Science, 177, 178–180. https://doi.org/10.1126/science.177.4044.178.
Zolnik, B. S., Gonzalez-Fernandez, A., Sadrieh, N., & Dobrovolskaia, M. A. (2010). Minireview: Nanoparticles and the immune system. Endocrinology, 151, 458–465.
Dobrovolskaia, M. A., Aggarwal, P., Hall, J. B., & McNeil, S. E. (2008). Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Molecular Pharmaceutics, 5, 487–495. https://doi.org/10.1021/mp800032f.
Akagi, T., M. Baba and M. Akashi, Biodegradable nanoparticles as vaccine adjuvants and delivery systems: regulation of immune responses by nanoparticle-based vaccine, in Polymers in nanomedicine. 2011, Springer. p. 31–64.
Tang, F., Li, L., & Chen, D. (2012). Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Advanced Materials, 24, 1504–1534. https://doi.org/10.1002/adma.201104763.
Stamopoulos, D., Gogola, V., Manios, E., Gourni, E., Benaki, D., Niarchos, D., & Pissas, M. (2009). Biocompatibility and solubility of Fe3O4-BSA conjugates with human blood. Current Nanoscience, 5, 177–181.
Acknowledgements
The instruction of Hongwu Du is thanked.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest. No human participant or Animal is involved in this review; thus, informed consent is not involved either.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, Z., Du, H. Prospect of Different Types of Magnetic Nanoparticles in Stem Cell Therapy. Stem Cell Rev and Rep 16, 675–683 (2020). https://doi.org/10.1007/s12015-020-09966-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-09966-9