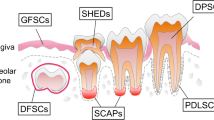
Abstract
Dental pulp stem cells (DPSC) are a heterogeneous population of highly proliferative stem cells located in the soft inner pulp tissue of the tooth. Demonstrated to have an affinity for neural differentiation, DPSC have been reported to generate functional Schwann cells (SC) through in vitro differentiation. Both DPSC and SC have neural crest origins, recently a significant population of DPSC have been reported to derive from peripheral nerve-associated glia. The predisposition DPSC have towards the SC lineage is not only a very useful tool for neural regenerative therapies in the medical field, it also holds great promise in the veterinary field. Devil Facial Tumour (DFT) is a clonally transmissible cancer of SC origin responsible for devastating wild populations of the Tasmanian devil. Very few studies have investigated the healthy Tasmanian devil SC (tdSC) for comparative studies between tdSC and DFT cells, and the development and isolation of a tdSC population is yet to be undertaken. A Tasmanian devil DPSC model offers a promising new outlook for DFT research, and the link between SC and DPSC may provide a potential explanation as to how a cancerous SC initially arose in a single Tasmanian devil to then go on to infect others as a parasitic clonal cell line. In this review we explore the current role of DPSC in human regenerative medicine, provide an overview of the Tasmanian devil and the devastating effect of DFT, and highlight the promising potential DPSC techniques pose for DFT research and our current understanding of DFT.


Similar content being viewed by others
Abbreviations
- DPSC:
-
dental pulp stem cell(s)
- SC:
-
Schwann cell(s)
- DFT:
-
Devil Facial Tumour
- tdSC:
-
Tasmanian devil Schwann cell(s)
- SCP:
-
Schwann cell precursor(s)
- MSC:
-
mesenchymal stem cell(s)
- ISCT:
-
International Society for Cellular Therapy
- NC:
-
neural crest
- BMMSC:
-
bone marrow mesenchymal stem cell(s)
- hDPSC:
-
adult human dental pulp stem cell(s)
- CNS:
-
central nervous system
- PNS:
-
peripheral nervous system
- CTVT:
-
Canine Transmissible Venereal Tumour
- CL:
-
Clam Leukaemia
- MHC:
-
major histocompatibility complex
- miRNA:
-
micro ribonucleic acid
- PRX:
-
periaxin
References
Gronthos, S., Mankani, M., Brahim, J., Robey, P. G., & Shi, S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America, 97(25), 13625–13630.
Rodriguez-Lozano, F. J., Bueno, C., Insausti, C. L., et al. (2011). Mesenchymal stem cells derived from dental tissues. International Endodontic Journal, 44, 800–806.
Janebodin, K., Horst, O. V., Ieronimakis, N., et al. (2011). Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS One, 6(11), e27526.
Stokowski, A., Shi, S., Sun, T., Bartold, P. M., Koblar, S. A., & Gronthos, S. (2007). EphB/Ephrin-B interaction mediates adult stem cell attachment, spreading, and migration: implications for dental tissue repair. Stem Cells, 25, 156–164.
Sonoyama, W., Yamaza, T., Gronthos, S., & Shi, H. (2008). Multipotent stem cells in dental pulp. In R. I. Freshney, G. N. Stacey, & J. M. Auerbach (Eds.), Culture of human stem cells (p. 187). Hoboken: Wiley.
Ferro, F., Spelat, R., & Baheney, C. S. (2014). Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. In C. Kioussi (Ed.), Stem Cells and Tissue Repair: Methods and Protocols 1210 (pp. 91–115). New York: Springer.
Tatullo, M., Marrelli, M., Shakesheff, K. M., & White, L. J. (2015). Dental pulp stem cells: function, isolation and applications in regenerative medicine. Journal of Tissue Engineering and Regenerative Medicine, 9(11), 1205–1216.
Al-Zer, H., Apel, C., Heiland, M., et al. (2015). Enrichment and schwann cell differentiation of neural crest-derived dental pulp stem cells. Vivo, 29(3), 319–326.
Martens, W., Sanen, K., Georgiou, M., et al. (2014). Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. The FASEB Journal, 28(4), 1634–1643.
Kaukua, N., Shahidi, M. K., Konstantinidou, C., et al. (2014). Glial origin of mesenchymal stem cells in a tooth model system. Nature, 513, 551–554.
Miletich, I., & Sharpe, P. T. (2004). Neural crest contribution to mammalian tooth formation. Birth Defects Research Part C: Embryo Today: Reviews, 72(2), 200–212.
Shi, H., Gong, Y., Qiang, L., et al. (2016). Derivation of Schwann cell precursors from neural crest cells resident in bone marrow for cell therapy to improve peripheral nerve regeneration. Biomaterials, 89, 25–37.
Al-Zer, H., & Kalbouneh, H. (2015). Dental pulp stem cells-derived schwann cells for peripheral nerve injury regeneration. Neural Regeneration Research, 10(12), 1945–1946.
Loh, R., Bergfeld, J., Hayes, D., et al. (2006). The pathology of devil facial tumor disease (DFTD) in Tasmanian devils (Sarcophilus harrisii). Veterinary Pathology, 43, 890–895.
Loh, R., Hayes, D., Mahjoor, A., O'Hara, A., Pyecroft, S., & Raidal, S. (2006). The immunohistochemical characterization of devil facial tumor disease (DFTD) in the Tasmanian devil (Sarcophilus harrisii). Veterinary Pathology, 43, 896–903.
Murchison, E. P., Tovar, C., Hsu, A., et al. (2010). The Tasmanian devil transcriptome reveals Schwann cell origins of a clonally transmissible cancer. Science, 327, 84–87.
Pearse, A. M., & Swift, K. (2006). Allograft theory: transmission of devil facial-tumour disease. Nature, 439, 549.
Bender, H. S. (2010). Devil Facial Tumour Disease (DFTD): Using genetics and genomics to investigate infectious disease in an endangered marsupial. In J. E. Deakin, P. D. Waters, & J. A. Marshall Graves (Eds.), Marsupial Genetics and Genomics (pp. 499–515). New York: Springer.
Welsh, J. S. (2011). Contagious cancer. The Oncologist, 16(1), 1–4.
Belov, K. (2012). Contagious cancer: Lessons from the devil and the dog. BioEssays, 34, 285–292.
McCallum, H., & Jones, M. (2012). Infectious cancers in wildlife. In A. A. Aguirre, R. S. Ostfeld, & P. Daszak (Eds.), New directions in conservation medicine: Applied cases of ecological health (pp. 270–283). New York: Oxford University Press, Inc..
Hass, R., Kasper, C., Bohm, S., & Jacobs, R. (2011). Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Communication and Signaling. https://doi.org/10.1186/1478-811X-9-12.
Friedenstein, A., Chailakhjan, R., & Lalykina, K. (1970). The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Proliferation, 3(4), 393–403.
Caplan, A. I. (1991). Mesenchymal stem cells. Journal of Orthopaedic Research, 9(5), 641–650.
Williams, A. R., & Hare, J. M. (2011). Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circulation Research, 109(8), 923–940.
Dominici, M., Le Blanc, K., Mueller, I., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317.
Horwitz, E. M., Le Blanc, K., Dominici, M., et al. (2005). Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy, 7(5), 393–395.
Sharpe, P. T. (2016). Dental mesenchymal stem cells. Development, 143(13), 2273–2280.
Seo, B. M., Miura, M., Gronthos, S., et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet, 364, 149–155.
Sonoyama, W., Liu, Y., Fang, D., et al. (2006). Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One, 1(1), e79.
Miura, M., Gronthos, S., Zhao, M., et al. (2003). SHED: stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences of the United States of America, 100(10), 5807–5812.
Volponi, A. A., & Sharpe, P. T. (2013). The tooth – a treasure chest of stem cells. British Dental Journal, 215(7), 353–358.
Mayo, V., Sawatari, Y., Huang, C. Y., & Garcia-Godoy, F. (2014). Neural crest-derived dental stem cells – Where we are and where we are going. Journal of Dentistry, 42(9), 1043–1051.
Gronthos, S., Brahim, J., Li, W., et al. (2002). Stem cell properties of human dental pulp stem cells. Journal of Dental Research, 81(8), 531–535.
Tovar, C., Pye, R. J., Kreiss, A., et al. (2017). Regression of devil facial tumour disease following immunotherapy in immunised Tasmanian devils. Scientific Reports. https://doi.org/10.1038/srep43827.
Sloan, A. J., & Smith, A. J. (2007). Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Diseases, 13(2), 151–157.
Huang, A. H. C., Snyder, B. R., Cheng, P. H., & Chan, A. W. S. (2008). Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells, 26, 2654–2663.
Huang, A. H. C., Chen, Y. K., Lin, L. M., Shich, T. Y., & Chan, A. W. S. (2008). Isolation and characterization of dental pulp stem cells from a supernumerary tooth. Journal of Oral Pathology & Medicine, 37, 571–574.
Huang, G. T., Yamaza, T., Shea, L. D., et al. (2010). Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Engineering: Part A, 16(2), 605–615.
d'Aquino, R., Papaccio, G., Laino, G., & Graziano, A. (2008). Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Reviews, 4(1), 21–26.
Huang, G. T., Gronthos, S., & Shi, S. (2009). Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. Journal of Dental Research, 88(9), 792–806.
Morad, G., Kheiri, L., & Khojasteh, A. (2013). Dental pulp stem cells for in vivo bone regeneration: A systematic review of literature. Archives of Oral Biology, 58, 1818–1827.
Govindasamy, V., Abdullah, A. N., Ronald, V. S., et al. (2010). Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. Journal of Endodontics, 36(9), 1504–1515.
Ellis, K. M., O'Carroll, D. C., Lewis, M. D., Rychkov, G. Y., & Koblar, S. A. (2014). Neurogenic potential of dental pulp stem cells isolated from murine incisors. Stem Cell Research & Therapy, 5(30), 1–13.
Arthur, A., Rychkov, G., Shi, S., Koblar, S. A., & Gronthos, S. (2008). Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells, 26(7), 1787–1795.
Leong, W. K., Henshall, T. L., Arthur, A., et al. (2012). Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms. Stem Cells Translational Medicine, 1(3), 177–187.
Huang, C. Y., Pelaez, D., Bendala, J. D., Garcia-Godoy, F., & Cheung, H. S. (2009). Plasiticity of stem cells derived from adut periodontal ligament. Regenerative Medicine, 4(6), 809–821.
Williams, M. L. K., & Solnica-Krezel, L. (2017). Regulation of gastrulation movements by emergent cell and tissue interactions. Current Opinion in Cell Biology, 48, 33–39.
Kaplan, K. M., Spivak, J. M., & Bendo, J. A. (2005). Embryology of the spine and associated congenital abnormalities. The Spine Journal, 5, 564–576.
Eyal-Giladi, H., & Kochav, S. (1976). From cleavage to primitive streak formation: A complementary normal table and a new look at the first stages of the development of the chick I. General morphology. Developmental Biology, 49(2), 321–337.
Chuai, M., Zeng, W., Yang, X., Boychenko, V., Glazier, J. A., & Weijer, C. J. (2006). Cell movement during chick primitive streak formation. Developmental Biology, 296, 137–149.
Weston, J. A. (1982). Regulation of neural crest cell migration and differentiation. In J. Gerhard (Ed.), Cell interactions and development (pp. 150–170). New York: Wiley.
Basch, M. L., Bronner-Fraser, M., & Garcia-Castro, M. I. (2006). Specification of the neural crest occurs during gastrulation and requires Pax7. Nature, 441, 218–222.
Le Douarin, N. M., & Kalcheim, C. (1999). The neural crest. Cambridge: Cambridge University Press.
Dupin, E., Creuzet, S., & Le Douarin, N. M. (2006). The contribution of the neural crest to the vertebrate body. Neural Crest Induction and Differentiation, 589, 96–119.
Le Douarin, N. M., & Dupin, E. (2003). Multipotency of the neural crest. Current Opinion in Genetics & Development, 13, 529–536.
Kruger, G. M., Mosher, J. T., Bixby, S., Joseph, N., Iwashita, T., & Morrison, S. J. (2002). Neural crest stem cells persist in the adult gut but undergo changes in self-renewal, neuronal subtype potential, and factor responsiveness. Neuron, 35, 657–669.
Fernandes, K. J., McKenzie, I. A., Mill, P., et al. (2004). A dermal niche for multipotent adult skin-derived precursor cells. Nature Cell Biology, 6, 1082–1093.
Sieber-Blum, M., Grim, M., Hu, Y. F., & Szeder, V. (2004). Pluripotent neural crest stem cells in the adult hair follicle. Developmental Dynamics, 231, 258–269.
Fernandes, K. J. L., Toma, J. G., & Miller, F. D. (2008). Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 363, 185–198.
Li, H. Y., Say, E. H. M., & Zhou, X. F. (2007). Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells, 25, 2053–2065.
Woodhoo, A., & Sommer, L. (2008). Development of the Schwann cell lineage: From the neural crest to the myelinated nerve. Glia, 56, 1481–1490.
Maro, G. S., Vermeren, M., Voiculescu, O., et al. (2004). Neural crest boundary cap cells constitute a sournce of neuronal and glial cells of the PNS. Nature Neuroscience, 7(9), 930–938.
Jones, M., Oakwood, M., Belcher, C. A., et al. (2003). Carnivore concerns: problems, issues and solutions for conserving Australasia’s marsupial carnivores. In M. Jones, C. Dickman, & M. Archer (Eds.), Predators with Pouches: The Biology of Carnivorous Marsupials (pp. 422–434). Collingwood: CSIRO.
Jones, M. E., Paetkau, D., Geffen, E., & Moritz, C. (2004). Genetic diversity and population structure of Tasmanian devils, the largest marsupial carnivore. Molecular Ecology, 13(8), 2197–2209.
Hawkins, C. E., Baars, C., Hesterman, H., et al. (2006). Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii. Biological Conservation, 131(2), 307–324.
Guiler, E. R. (1970). Obsevations on the Tasmanian devil, Sarcophilus harrisii (Marsupialia: Dasyuridae) I. Numbers, home range, movements, and food in two populations. Australian Journal of Zoology, 18, 49–62.
Pemberton, D. (1990). Social organisation and behaviour of the Tasmanian devil, Sarcophilus harrisii. PhD Thesis, Tasmania: University of Tasmania.
Jones, M. E., Cockburn, A., Hamede, R., et al. (2008). Life-history change in disease-ravaged Tasmanian devil populations. Proceedings of the National Academy of Sciences of the United States of America, 105(29), 10023–10027.
McCallum, H., & Jones, M. (2006). To lose both would look like carelessness: Tasmanian devil facial tumour disease. PLoS Biology, 4(10), e342.
Pyecroft, S. B., Pearse, A., Loh, R., et al. (2007). Towards a case definition for devil facial tumour disease: What is it? EcoHealth, 4(3), 346–351.
Siddle, H. V., Kreiss, A., Eldridge, M. D., et al. (2007). Transmission of a fatal clonal tumor by biting occurs due to depleted MHC diversity in a threatened carnivorous marsupial. Proceedings of the National Academy of Sciences of the United States of America, 104(41), 16221–16226.
Griner, L. A. (1979). Neoplasms in Tasmanian devils (Sarcophilus harrisii). Journal of the National Cancer Institute, 62(3), 589–595.
Canfield, P. J., Hartley, W. J., & Reddacliff, G. L. (1990). Spontaneous proliferations in Australian marsupials - a survey and review. 1. Macropods, koalas, wombats, possums and gliders. Journal of Comparative Pathology, 103, 135–146.
Metzger, M. J., & Goff, S. P. (2016). A sixth modality of infectious disease: Contagious cancer from devils to clams and beyond. PLoS Pathogens, 12(10), e1005904.
Ostrander, E. A., Davis, B. W., & Ostrander, G. K. (2016). Transmissible tumors: Breaking the cancer paradigm. Trends in Genetics, 32(1), 1–15.
Murgia, C., Pritchard, J. K., Kim, S. Y., Fassati, A., & Weiss, R. A. (2006). Clonal origin and evolution of a transmissible cancer. Cell, 126(3), 477–487.
Rebbeck, C. A., Thomas, R., Breen, M., Leroi, A. M., & Burt, A. (2009). Origins and evolution of a transmissible cancer. Evolution, 63(9), 2340–2349.
Carballal, M. J., Barber, B. J., Iglesias, D., & Villalba, A. (2015). Neoplastic diseases of marine bivalves. Journal of Invertebrate Pathology, 131, 83–106.
Böttger, S. A., Amarosa, E. J., Geoghegan, P., & Walker, C. W. (2013). Chronic natural occurrence of disseminated neoplasia in select populations of the soft-shell clam, Mya arenaria, in New England. Northeastern Naturalist, 20(3), 430–440.
Hamede, R. K., McCallum, H., & Jones, M. (2008). Seasonal, demographic and density-related patterns of contact between Tasmanian devils (Sarcophilus harrisii): Implications for transmission of devil facial tumour disease. Austral Ecology, 33(5), 614–622.
Woods, G. M., Kreiss, A., Belov, K., Siddle, H. V., Obendorf, D. L., & Muller, H. K. (2007). The immune response of the Tasmanian devil (Sarcophilus harrisii) and Devil Facial Tumour Disease. EcoHealth, 4(3), 338–345.
Ujvari, B., & Belov, K. (2011). Major histocompatibility complex (MHC) markers in conservation biology. International Journal of Molecular Sciences, 12, 5168–5186.
Kreiss, A., Tovar, C., Obendorf, D. L., Dun, K., & Woods, G. M. (2011). A murine xenograft model for a transmissible cancer in Tasmanian devils. Veterinary Pathology, 48(2), 475–481.
Kreiss, A., Cheng, Y., Kimble, F., et al. (2011). Allorecognition in the Tasmanian devil (Sarcophilus harrisii), an endangered marsupial species with limited genetic diversity. PLoS One, 6(7), e22402.
Siddle, H. V., Kreiss, A., Tovar, C., et al. (2013). Reversible epigenetic down-regulation of MHC molecules by devil facial tumour disease illustrates immune escape by a contagious cancer. Proceedings of the National Academy of Sciences of the United States of America, 110(13), 5103–5108.
Hartline, D. K. (2008). What is myelin? Neuron Glia Biology, 4(2), 153–163.
Tovar, C., Obendorf, D., Murchison, E. P., Papenfuss, A. T., Kreiss, A., & Woods, G. M. (2011). Tumor-specific diagnostic marker for transmissible facial tumors of Tasmanian devils: immunohistochemistry studies. Veterinary Pathology, 48(6), 1195–1203.
Burnett, M. G., & Zager, E. L. (2004). Pathophysiology of peripheral nerve injury: a brief review. Neurosurgical Focus, 16(5), 1–7.
Waller, A. (1850). Experiments on the section of glossopharangeal and hypoglossal nerves of the frog and observations of the alternatives produced thereby in the structure of their primitive fibres. Philosophical Transactions of the Royal Society B: Biological Sciences, 140, 423–429.
Coleman, M. P., & Freeman, M. R. (2010). Wallerian degeneration, Wlds, and Nmnat. Annual Review of Neuroscience, 33, 245–267.
Wei, Y., Zhou, J., Zheng, Z., et al. (2009). An improved method for isolating Schwann cells from postnatal rat sciatic nerves. Cell and Tissue Research, 337, 361–369.
Acknowledgements
The authors acknowledge the Sir Mark Mitchell Research Foundation and the University of Adelaide, School of Animal and Veterinary Sciences, for providing funding support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Graham, C.M., Kremer, K.L., Koblar, S.A. et al. Dental Pulp Stem Cells - Exploration in a Novel Animal Model: the Tasmanian Devil (Sarcophilus harrisii). Stem Cell Rev and Rep 14, 500–509 (2018). https://doi.org/10.1007/s12015-018-9814-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-018-9814-0