Abstract
Due to their extraordinarily broad differentiation potential and persistence during adulthood, adult neural crest-derived stem cells (NCSCs) are highly promising candidates for clinical applications, particularly when facing the challenging treatment of neurodegenerative diseases or complex craniofacial injuries. Successful application of human NCSCs in regenerative medicine and pharmaceutical research mainly relies on the availability of sufficient amounts of tissue for cell isolation procedures. Facing this challenge, we here describe for the first time a novel population of NCSCs within the middle turbinate of the human nasal cavity. From a surgical point of view, high amounts of tissue are routinely and easily removed during nasal biopsies. Investigating the presence of putative stem cells in obtained middle turbinate tissue by immunohistochemistry, we observed Nestin+/p75NTR+/S100+/α smooth muscle actin (αSMA)− cells, which we successfully isolated and cultivated in vitro. Cultivated middle turbinate stem cells (MTSCs) kept their expression of neural crest and stemness markers Nestin, p75 NTR and S100 and showed the capability of sphere formation and clonal growth, indicating their stem cell character. Application of directed in vitro differentiation assays resulted in successful differentiation of MTSCs into osteogenic and neuronal cell types. Regarding the high amount of tissue obtained during surgery as well as their broad differentiation capability, MTSCs seem to be a highly promising novel neural crest stem cell population for applications in cell replacement therapy and pharmacological research.



Similar content being viewed by others
References
Wagers, A. J., & Weissman, I. L. Plasticity of adult stem cells. Cell 2004;116:639 – 48.
Schofield, R. (1978). The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells, 4, 7–25.
Collins, C. A., Olsen, I., Zammit, P. S., et al. (2005). Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell, 122, 289–301.
Clewes, O., Narytnyk, A., Gillinder, K. R., Loughney, A. D., Murdoch, A. P., & Sieber-Blum, M. Human epidermal neural crest stem cells (hepi-ncsc)-characterization and directed differentiation into osteocytes and melanocytes. Stem Cell Reviews 2011.
Dupin, E., & Sommer, L. (2012). Neural crest progenitors and stem cells: from early development to adulthood. Developmental Biology, 366, 83–95.
Murrell, W., Feron, F., Wetzig, A., et al. (2005). Multipotent stem cells from adult olfactory mucosa. Developmental Dynamics, 233, 496–515.
Müller, J., Ossig, C., Greiner, J. F., et al. (2015). Intrastriatal transplantation of adult human neural crest-derived stem cells improves functional outcome in Parkinsonian rats. Stem Cells Translational Medicine, 4, 31–43.014.
His, W. (1868). Untersuchungen über die erste Anlage des Wirbeltierleibes. Die erste Entwicklung des Hühnchens im Ei. Leipzig: Vogel.
Kaltschmidt, B., Kaltschmidt, C., & Widera, D. (2012). Adult craniofacial stem cells: sources and relation to the neural crest. Stem Cell Reviews, 8, 658 – 71.
Toma, J. G., Akhavan, M., Fernandes, K. J., et al. (2001). Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nature Cell Biology, 3, 778 – 84.
Hauser, S., Widera, D., Qunneis, F., et al. (2012). Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells and Development, 21, 742 – 56.
Tabakow, P., Raisman, G., Fortuna, W., et al. Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplantation 2014.
Arthur, A., Rychkov, G., Shi, S., Koblar, S. A., & Gronthos, S. (2008). Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells, 26, 1787–1795.
Nakashima, T., Kimmelman, C. P., & Snow, J. B. Jr. (1984). Structure of human fetal and adult olfactory neuroepithelium. Archives of Otolaryngology, 110, 641–646.
Feron, F., Perry, C., Cochrane, J., et al. (2005). Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain, 128, 2951–2960.
Murrell, W., Wetzig, A., Donnellan, M., et al. (2008). Olfactory mucosa is a potential source for autologous stem cell therapy for Parkinson’s disease. Stem Cells, 26, 2183–2192.
Mackay-Sim, A. (2005). Olfactory ensheathing cells and spinal cord repair. The Keio Journal of Medicine, 54, 8–14.
Greiner, J. F., Hauser, S., Widera, D., et al. (2011). Efficient animal-serum free 3D cultivation method for adult human neural crest-derived stem cell therapeutics. European Cell & Materials, 22, 403 – 19.
Hofemeier, A. D., Hachmeister, H., Pilger, C., et al. (2016). Label-free nonlinear optical microscopy detects early markers for osteogenic differentiation of human stem cells. Scientific Reports, 6, 26716.
Muller, J., Greiner, J. F., Zeuner, M., et al. (2016). 1,8-Cineole potentiates IRF3-mediated antiviral response in human stem cells and in an ex vivo model of rhinosinusitis. Clinical Science (London), 130, 1339–1352.
Delorme, B., Nivet, E., Gaillard, J., et al. (2010). The human nose harbors a niche of olfactory ectomesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells and Development, 19, 853 – 66.
Damm, M., Vent, J., Schmidt, M., et al. (2002). Intranasal volume and olfactory function. Chemical Senses, 27, 831–839.
Barnett, S. C., Alexander, C. L., Iwashita, Y., et al. (2000). Identification of a human olfactory ensheathing cell that can effect transplant-mediated remyelination of demyelinated CNS axons. Brain, 123(Pt 8), 1581–1588.
Barraud, P., Seferiadis, A. A., Tyson, L. D., et al. (2010)Neural crest origin of olfactory ensheathing glia. Proceedings of the National Academy of Sciences of the United States of America ;107:21040–5.
Viktorov, I. V., Savchenko, E. A., & Chekhonin, V. P. (2007). Spontaneous neural differentiation of stem cells in culture of human olfactory epithelium. Bulletin of Experimental Biology and Medicine, 144, 596–601.
Jahed, A., Rowland, J. W., McDonald, T., Boyd, J. G., Doucette, R., & Kawaja, M. D. (2007). Olfactory ensheathing cells express smooth muscle alpha-actin in vitro and in vivo. The Journal of Comparative Neurology, 503, 209 – 23.
Nivet, E., Vignes, M., Girard, S. D., et al. (2011). Engraftment of human nasal olfactory stem cells restores neuroplasticity in mice with hippocampal lesions. The Journal of Clinical Investigation, 121, 2808–2820.
Dayal, A., Rhee, J. S., & Garcia, G. J. (2016). Impact of middle versus inferior total turbinectomy on nasal aerodynamics. Otolaryngology–Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and. Neck Surgery, 155, 518 – 25.
Scheithauer, M. O. (2010). Surgery of the turbinates and “empty nose” syndrome. GMS Current Topics Otorhinolaryngol Head Neck Surgery, 9, Doc03.
Modi, P., & Rahamim, J. (2005). Fibrin sealant treatment of splenic injuries during oesophagectomy. European Journal Cardiothoracic Surgery, 28, 167–168.
Dahlstrom, K. K., Weis-Fogh, U. S., Medgyesi, S., Rostgaard, J., & Sorensen, H. The use of autologous fibrin adhesive in skin transplantation. Plastic and Reconstructive Surgery 1992;89:968 – 72; discussion 73 – 6.
Gerard, C., Forest, M. A., Beauregard, G., Skuk, D., & Tremblay, J. P. (2012). Fibrin gel improves the survival of transplanted myoblasts. Cell Transplantation, 21(1), 127 – 37.
Ho, W., Tawil, B., Dunn, J. C., & Wu, B. M. (2006). The behavior of human mesenchymal stem cells in 3D fibrin clots: dependence on fibrinogen concentration and clot structure. Tissue Engineering, 12, 1587–1595.
Peterbauer-Scherb, A., Danzer, M., Gabriel, C., van Griensven, M., Redl, H., & Wolbank, S. (2012). In vitro adipogenesis of adipose-derived stem cells in 3D fibrin matrix of low component concentration. Journal Tissue of Engineering and Regenerative Medicine, 6, 434 – 42.
Greiner, J. F., Grunwald, L. M., Muller, J., et al. (2014). Culture bag systems for clinical applications of adult human neural crest-derived stem cells. Stem Cell Research & Therapy, 5, 34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
We have no conflicts of interest relevant to the content of this article. Human nasal middle turbinates and adipose tissue were obtained via routine nasal surgery after informed written consent according to local and international guidelines (Bezirksregierung Detmold/Münster). Isolation and further experimental procedures were ethically approved by the ethics commission of the Ärztekammer Westfalen-Lippe and the medical faculty of the Westfälische Wilhems-Universität (Münster, Germany).
Electronic supplementary material
Below is the link to the electronic supplementary material.

12015_2017_9797_MOESM1_ESM.jpg
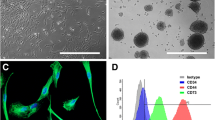
Supplementary figure S1: Isolated hMSCs show characteristic morphology, expression profile and differentiation capability into mesodermal cell types. (A)Isolated MSCs show characteristic morphology. (B) RT-PCR and qPCR-analysis revealed the expression of MSC markers CD105, CD106, CD90, CD29, CD73 while lacking expression of non-MSC markers CD45 and CD13. (C) Expression of CD105 in isolated hMSCs was validated on protein level using flow cytometry. (D) hMSCs were able to differentiate into osteogenic cell types visualized by Alizarin red S-stained calcium deposition and adipogenic cells containing Oil red O-positive lipid droplets. (JPG 4956 KB)
Rights and permissions
About this article
Cite this article
Schürmann, M., Brotzmann, V., Bütow, M. et al. Identification of a Novel High Yielding Source of Multipotent Adult Human Neural Crest-Derived Stem Cells. Stem Cell Rev and Rep 14, 277–285 (2018). https://doi.org/10.1007/s12015-017-9797-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-017-9797-2