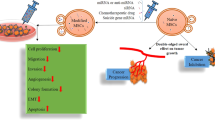
Abstract
MicroRNAs (miRs) are potential therapeutic targets in glioblastoma multiforme (GBM), but the difficulties associated with their delivery to tumor target cells have hampered their widespread use. Mesenchymal stem cells (MSCs) can migrate to the sites of cancers, including GBM and exert anti-tumor effects. In this study, it is shown that Wharton’s jelly-MSCs (WJ-MSCs) have the ability to deliver exogenous miRs to GBM cells and the functional impact of this delivery is characterized. It is found that the labeled miR-124, as an example for miR of interest, can be successfully delivered with WJ-MSCs to U87 GBM cells via dependent or exosome-independent processes. It is demonstrated that the delivered exogenous miR-124 significantly decreases the luciferase activity of the target gene CDK6. In addition, the delivered miR-124 enhances the chemosensitivity of GBM cells to temozolomide and decreases the migration of GBM cells. These results suggest that the use of exogenous miRNA delivery with the derived exosomes from WJ-MSCs may provide a novel approach for miRNA replacement therapy in GBM cancers.






Similar content being viewed by others
References
Furnari, F. B., Fenton, T., Bachoo, R. M., et al. (2007). Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes & Development, 21, 2683–2710.
Desjardins, A., Rich, J. N., Quinn, J. A., et al. (2005). Chemotherapy and novel therapeutic approaches in malignant glioma. Frontiers in Bioscience, 10, 2645–2668.
Giese, A., Bjerkvig, R., Berens, M., & Westphal, M. (2003). Cost of migration: invasion of malignant gliomas and implications for treatment. Journal of Clinical Oncology, 21, 1624–1636.
Louis, D., Posner, J., Jacobs, T., & Kaplan, R. (2000). Report of the brain tumor progress review group. Leesburg: National Institute of Neurological Disorders and Stroke, pp. 1–96.
Yamada, R., & Nakano, I. (2012). Glioma stem cells: their role in chemoresistance. World Neurosurgery, 77, 237–240.
Nakamizo, A., Marini, F., Amano, T., et al. (2005). Human bone marrow–derived mesenchymal stem cells in the treatment of gliomas. Cancer Research, 65, 3307–3318.
Abba, M., Mudduluru, G., & Allgayer, H. (2012). MicroRNAs in cancer: small molecules, big chances. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 12, 733–743.
Li, Y., Guessous, F., Zhang, Y., et al. (2009). MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Research, 69, 7569–7576.
Godlewski, J., Nowicki, M. O., Bronisz, A., et al. (2008). Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Research, 68, 9125–9130.
Bao, L., Hazari, S., Mehra, S., Kaushal, D., Moroz, K., & Dash, S. (2012). Increased expression of P-glycoprotein and doxorubicin chemoresistance of metastatic breast cancer is regulated by miR-298. The American Journal of Pathology, 180, 2490–2503.
Lawler, S., & Chiocca, E. A. (2009). Emerging functions of microRNAs in glioblastoma. Journal of Neuro-Oncology, 92, 297–306.
An, L., Liu, Y., Wu, A., & Guan, Y. (2013). microRNA-124 inhibits migration and invasion by down-regulating ROCK1 in glioma. PLoS One, 8, e69478.
Wei, J., Wang, F., Kong, L. Y., et al. (2013). MiR-124 inhibits STAT3 signaling to enhance T cell–mediated immune clearance of glioma. Cancer Research, 73, 3913–3926.
Chen, S. M., Chou, W. C., Hu, L. Y., et al. (2015). The effect of MicroRNA-124 over expression on anti-tumor drug sensitivity. PLoS One, 10, e0128472.
Fowler, A., Thomson, D., Giles, K., et al. (2011). miR-124a is frequently down-regulated in glioblastoma and is involved in migration and invasion. European Journal of Cancer, 47, 953–963.
Silber, J., Lim, D. A., Petritsch, C., et al. (2008). miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Medicine, 6, 14.
Shi, Z., Chen, Q., Li, C., et al. (2014). MiR-124 governs glioma growth and angiogenesis and enhances chemosensitivity by targeting R-Ras and N-Ras. Neuro-Oncology, 16(10), 1341–1353.
Shah, K. (2012). Mesenchymal stem cells engineered for cancer therapy. Advanced Drug Delivery Reviews, 64, 739–748.
Sun, X. Y., Nong, J., Qin, K., Warnock, G. L., & Dai, L. J. (2011). Mesenchymal stem cell-mediated cancer therapy: a dual-targeted strategy of personalized medicine. World Journal Stem Cells, 3, 96–103.
Kim, D. W., Staples, M., Shinozuka, K., Pantcheva, P., Kang, S. D., & Borlongan, C. V. (2013). Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. International Journal of Molecular Sciences, 14, 11692–11712.
Hsieh, J. Y., Wang, H. W., Chang, S. J., et al. (2013). Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PloS One, 8, e72604.
Liu, S., Hou, K. D., Yuan, M., et al. (2014). Characteristics of mesenchymal stem cells derived from Wharton’s jelly of human umbilical cord and for fabrication of non-scaffold tissue-engineered cartilage. Journal of Bioscience and Bioengineering, 117, 229–235.
Ng, F., Boucher, S., Koh, S., et al. (2008). PDGF, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood, 112, 295–307.
Pittenger, M. F., Mackay, A. M., Beck, S. C., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284, 143–147.
Greco, S. J., & Rameshwar, P. (2012). Mesenchymal stem cells in drug/gene delivery: implications for cell therapy. Therapeutic Delivery, 3, 997–1004.
Matuskova, M., Hlubinova, K., Pastorakova, A., et al. (2010). HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Letters, 290, 58–67.
Mittelbrunn, M., Gutiérrez-Vázquez, C., Villarroya-Beltri, C., et al. (2011). Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature Communications, 2, 282.
Kosaka, N., Iguchi, H., Yoshioka, Y., Takeshita, F., Matsuki, Y., & Ochiya, T. (2010). Secretory mechanisms and intercellular transfer of microRNAs in living cells. Journal of Biological Chemistry, 285, 17442–17452.
Collino, F., Deregibus, M. C., Bruno, S., et al. (2010). Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One, 5, e11803.
Broderick, J. A., & Zamore, P. D. (2011). MicroRNA therapeutics. Gene Therapy, 18, 1104–1110.
Karussis, D., Kassis, I., Kurkalli, B. G. S., & Slavin, S. (2008). Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. Journal of the Neurological Sciences, 265, 131–135.
Mohr, A., Lyons, M., Deedigan, L., et al. (2008). Mesenchymal stem cells expressing TRAIL lead to tumour growth inhibition in an experimental lung cancer model. Journal of Cellular and Molecular Medicine, 12, 2628–2643.
Amano, S., Li, S., Gu, C., et al. (2009). Use of genetically engineered bone marrow-derived mesenchymal stem cells for glioma gene therapy. International Journal of Oncology, 35, 1265.
Park, S. A., Ryu, C. H., Kim, S. M., et al. (2011). CXCR4-transfected human umbilical cord blood-derived mesenchymal stem cells exhibit enhanced migratory capacity toward gliomas. International Journal of Oncology, 38, 97.
Lim, P. K., Bliss, S. A., Patel, S. A., et al. (2011). Gap junction–mediated import of MicroRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Research, 71, 1550–1560.
Bader, A. G., Brown, D., & Winkler, M. (2010). The promise of microRNA replacement therapy. Cancer Research, 70, 7027–7030.
Nana-Sinkam, S. P., & Croce, C. M. (2011). MicroRNAs as therapeutic targets in cancer. Translational Research, 157, 216–225.
Yoo, A. S., Sun, A. X., Li, L., et al. (2011). MicroRNA-mediated conversion of human fibroblasts to neurons. Nature, 476, 228–231.
Cheng, L. C., Pastrana, E., Tavazoie, M., & Doetsch, F. (2009). miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nature Neuroscience, 12, 399–408.
Lee, H. K., Finniss, S., Cazacu, S., et al. (2013). Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget, 4, 346–361.
Katakowski, M., Buller, B., Zheng, X., et al. (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Letters, 335, 201–204.
Munoz, J. L., Bliss, S. A., Greco, S. J., Ramkissoon, S. H., Ligon, K. L., & Rameshwar, P. (2013). Delivery of functional anti-miR-9 by mesenchymal stem cell–derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Molecular Therapy—Nucleic Acids, 2, e126.
Blum, R., Jacob-Hirsch, J., Amariglio, N., Rechavi, G., & Kloog, Y. (2005). Ras inhibition in glioblastoma down-regulates hypoxia-inducible factor-1α, causing glycolysis shutdown and cell death. Cancer Research, 65, 999–1006.
Zou, C., Xu, Q., Mao, F., et al. (2012). MiR-145 inhibits tumor angiogenesis and growth by N-RAS and VEGF. Cell Cycle, 11, 2137–2145.
Ryan, J. M., Barry, F. P., Murphy, J. M., & Mahon, B. P. (2005). Mesenchymal stem cells avoid allogeneic rejection. Journal of Inflammation, 2, 8.
Pileggi, A., Xu, X., Tan, J., & Ricordi, C. (2013). Mesenchymal stromal (stem) cells to improve solid organ transplant outcome: lessons from the initial clinical trials. Current Opinion in Organ Transplantation, 18, 672.
Wang, H. S., Hung, S. C., Peng, S. T., et al. (2004). Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells, 22, 1330–1337.
Drela, K., Lech, W., Figiel-Dabrowska, A., et al. (2016). Enhanced neuro-therapeutic potential of Wharton’s Jelly–derived mesenchymal stem cells in comparison with bone marrow mesenchymal stem cells culture. Cytotherapy, 18, 497–509.
de Castro, J. V., Gomes, E. D., Granja, S., et al. (2017). Impact of mesenchymal stem cells’ secretome on glioblastoma pathophysiology. Journal of Translational Medicine, 15, 200.
Wang, B., Yao, K., Huuskes, B. M., et al. (2016). Mesenchymal stem cells deliver exogenous microRNA-let7c via exosomes to attenuate renal fibrosis. Molecular Therapy, 24, 1290–1301.
Li, X., Liu, L., Yang, J., et al. (2016). Exosome derived from human umbilical cord mesenchymal stem cell mediates mir-181c attenuating burn-induced excessive inflammation. EBioMedicine, 8, 72–82.
Acknowledgements
This work is a part of PhD thesis of Samaneh Sharif that is financially supported by the Department of Molecular Medicine, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
The authors indicate no potential conflicts of interest. All the authors have materially participated in the research and the article preparation and have approved the final article.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Sharif, S., Ghahremani, M.H. & Soleimani, M. Delivery of Exogenous miR-124 to Glioblastoma Multiform Cells by Wharton’s Jelly Mesenchymal Stem Cells Decreases Cell Proliferation and Migration, and Confers Chemosensitivity. Stem Cell Rev and Rep 14, 236–246 (2018). https://doi.org/10.1007/s12015-017-9788-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-017-9788-3