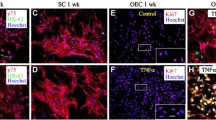
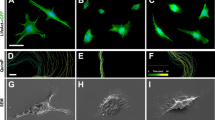
Abstract
Olfactory Ensheathing Cells (OECs), exhibiting phenotypic characteristics of both astrocytes and Schwann Cells, show peculiar plasticity. In vitro, OECs promote axonal growth, while in vivo they promote remyelination of damaged axons. We decided to further investigate OEC potential for regeneration and functional recovery of the damaged Central Nervous System (CNS). To study OEC antigen modulation, OECs prepared from postnatal mouse olfactory bulbs were grown in different culture conditions: standard or serum-free media with/without Growth Factors (GFs) and analyzed for different neural specific markers. OEC functional characterizations were also achieved. Resistance of OECs to the neurotoxin 6-hydroxydopamine (6-OHDA) was analyzed by evaluating apoptosis and death. OEC neuroprotective properties were investigated by in vitro co-cultures or by addition of OEC conditioned medium to the neuroblastoma SH-SY5Y cells exposed to 6-OHDA. We observed: 1) modification of OEC morphology, reduced cell survival and marker expression in serum-free medium; 2) GF addition to serum-free medium condition influenced positively survival and restored basal marker expression; 3) no OEC apoptosis after a prolonged exposition to 6-OHDA; 4) a clear OEC neuroprotective tendency, albeit non statistically significant, on 6-OHDA treated SH-SY5Y cells. These peculiar properties of OECs might render them potential clinical agents able to support injured CNS.





Similar content being viewed by others
References
Graziadei, P. P. C., & Monti-Graziadei, G. A. (1979). Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological Aspects of Differentiation and Structural Organization of the Olfactory Sensory Neurons. Journal of Neurocytology, 8, 1–18.
Fairless, R., & Barnett, S. (2005). Olfactory ensheathing cells: their role in central nervous system repair. The International Journal of Biochemistry and Cell Biology, 37, 693–699.
Doucette, R. (1990). Glial influences on axonal growth in the primary olfactory system. Glia, 3, 433–449.
Golgi, C. (1875). Sulla fina anatomia dei bulbi olfattorii. Rivista Sperimentale di Freniatria, 1, 403–425.
Blanes, T. (1898). Sobre algunos puntos dudosos de la estructura del bulbo olfatorio. Revista Trimestral Micrografica, 3, 99–127.
Ramon-Cueto, A., & Avila, J. (1998). Olfactory ensheathing cells: properties and function. Brain Research Bulletin, 46, 175–187.
Wewetzer, K., Verdù, E., Angelov, D. N., & Navarro, X. (2002). Olfactory ensheathing glia and Schwann cells: two of a kind? Cellular Tissue Research, 309, 337–345.
Van den Pol, A. N., & Santarelli, J. G. (2003). Olfactory ensheathing cells: time lapse imaging of cellular interactions, axonal support, rapid morphologic shifts, and mitosis. Journal of Comparative Neurology, 458, 175–194.
Yang, H., He, B. R., & Hao, D. J. (2014). Biological roles of olfactory ensheathing cells in facilitating neural regeneration: a systematic review. Molecular Neurobiology, 51(1), 168–179.
Lipson, A. C., Widenfalk, J., Lindqvist, E., Ebendal, T., & Olson, L. (2003). Neurotrophic properties of olfactory ensheathing glia. Experimental Neurology, 180, 167–171.
Mackay-Sim, A., & Chuah, M. I. (2000). Neurotrophic factors in the primary olfactory pathway. Progress in Neurobiology, 62, 527–559.
Woodhall, E., West, A. K., & Chuah, M. I. (2001). Cultured olfactory ensheathing cells express nerve growth factor, brain-derived neurotrophic factor, glia cell line-derived neurotrophic factor and their receptors. Molecular Brain Research, 88, 203–213.
Wewetzer, K., Grothe, C., & Claus, P. (2001). In vitro expression and regulation of ciliary neurotrophic factor and its α receptor subunit in neonatal rat olfactory ensheathing cells. Neuroscience Letters, 306, 165–168.
Boruch, A. V., Conners, J. J., Pipitone, M., Deadwyler, G., Storer, P. D., Devries, et al. (2001). Neurotrophic and migratory properties of an olfactory ensheathing cell line. Glia, 33, 225–229.
Doucette, R. (1996). Immunohistochemical localization of laminin, fibronectin and collagen type IV in the nerve fiber layer of the olfactory bulb. International Journal of Developmental Neuroscience, 14, 945–959.
Franceschini, I. A., & Barnett, S. C. (1996). Low-affinity NGF-receptor and E-N-CAM expression define two types of olfactory nerve ensheathing cells that share a common lineage. Developmental Biology, 173, 327–343.
Pastrana, E., Moreno-Flores, M. T., Gurzov, E. N., Ávila, J., Wandosell, F., & Díaz-Nido, J. (2006). Genes associated with adult axon regeneration promoted by olfactory ensheathing cells: a new role for matrix metalloproteinase 2. Journal of Neuroscience, 26, 5347–5359.
Moreno-Flores, M. T., Lim, F., Martín-Bermejo, M. J., Díaz-Nido, J., Ávila, J., & Wandosell, F. (2003). High level of amyloid precursor protein expression in neurite-promoting olfactory ensheathing glia (OEG) and OEG-derived cell lines. Journal of Neuroscience Research, 71, 871–881.
Barnett, S. C., & Riddell, J. S. (2004). Olfactory ensheathing cells (OECs) and the treatment of CNS injury: advantages and possible caveats. Journal of Anatomy, 204, 57–67.
Franssen, E. H., de Bree, F. M., & Verhaagen, J. (2007). Olfactory ensheathing glia: their contribution to primary olfactory nervous system regeneration and their regenerative potential following transplantation into the injured spinal cord. Brian Research, 56, 236–258.
Raisman, G. (2001). Olfactory ensheathing cells – another miracle cure for spinal cord injury? Nature Reviews Neuroscience, 2, 369–375.
Ramon-Cueto, A., Corsero, M. I., Santos-Benito, F. F., & Avila, J. (2000). Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing cells. Neuron, 25, 425–435.
Lee, M. J., Calle, E., Brennan, A., Ahmed, S., Sviderskaya, E., Jessen, K. R., et al. (2001). In early development of the rat mRNA for the major myelin protein P(0) is expressed in non-sensory areas of the embryonic inner ear, notochord, enteric nervous system, and olfactory ensheathing cells. Developmental Dynamics, 222, 40–51.
Franklin, R. J. M. (2003). Remyelination by transplanted olfactory ensheathing cells. The Anatomical Record, 271B, 71–76.
Sasaki, M., Lankford, K. L., Radtke, C., Honmou, O., & Kocsis, J. D. (2011). Remyelination after olfactory ensheathing cell transplantation into diverse demyelinating environments. Experimental Neurology, 229(1), 88–98.
Sasaki, M., Hains, B., Lankford, K. L., Waxman, S. G., & Kocsis, J. D. (2006). Protection of corticospinal tract neurons after dorsal spinal cord transaction and engraftment of olfactory ensheathing cells. Glia, 53, 352–359.
Oudega, M., & Xu, X. M. (2006). Schwann cell transplantation for repair of the adult spinal cord. Journal of Neurotrauma, 23, 453–467.
Lindsay, S. L., Riddell, J. S., & Barnett, S. C. (2010). Olfactory mucosa for transplant mediated repair: a complex tissue for a complex injury? Glia, 58, 125–134.
Raisman, G., & Li, Y. (2007). Repair of neural pathways by olfactory ensheathing cells. Nature Reviews Neuroscience, 8, 312–319.
Chehrehasa, F., Windus, L. C., Ekberg, J. A., Scott, S. E., Amaya, D., Mackay-Sim, A., et al. (2010). Olfactory glia enhance neonatal axon regeneration. Molecular and Cellular Neuroscience, 45(3), 277–288.
Su, Z., & He, C. (2010). Olfactory ensheathing cells: biology in neural development and regeneration. Progress in Neurobiology, 92(4), 517–532.
Cova, L., Armentero, M. T., Zennaro, E., Calzarossa, C., Bossolasco, P., Busca G., et al. (2010). Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson’s disease. Brain Research, 1311, 12–27.
Blandini, F., Cova, L., Armentero, M. T., Zennaro, E., Levandis, G., Bossolasco, P., et al. (2010). Transplantation of undifferentiated human mesenchymal stem cells protects against 6-hydroxydopamine neurotoxicity in the rat. Cell Transplantation, 19(2), 203–217.
Ubink, R., & Hökfelt, T. (2000). Expression of neuropeptide Y in olfactory ensheathing cells during prenatal development. Journal of Comparative Neurology, 423, 13–25.
Ebel, C., Brandes, G., Radtke, C., Rohn, K., & Wewetzer, K. (2013). Clonal in vitro analysis of neurotrophin receptor p75-immunofluorescent cells reveals phenotypic plasticity of primary rat olfactory ensheathing cells. Neurochemistry Research, 38(5), 1078–1087.
Vincent, A. J., West, A. K., & Chuah, M. I. (2005). Morphological and functional plasticity of olfactory ensheathing cells. Journal of Neurocytology, 34, 65–80.
Pellitteri, R., Spatuzza, M., Russo, A., Zaccheo, D., & Stanzani, S. (2009). Olfactory ensheathing cells represent an optimal substrate for hippocampal neurons: an in vitro study. International Journal of Developmental Neuroscience, 27(5), 453–458.
Chuah, M. I., & Au, C. (1993). Cultures of ensheathing cells from neonatal rat olfactory bulbs. Brain Research, 601(1–2), 213–220.
Cova, L., Bossolasco, P., Armentero, M. T., Diana, V., Zennaro, E., Mellone, M., et al. (2012). Neuroprotective effects of human mesenchymal stem cells on neural cultures exposed to 6-hydroxydopamine: implications for reparative therapy in Parkinson’s disease. Apoptosis, 17(3), 289–304.
Pellitteri, R., Spatuzza, M., Stanzani, S., & Zaccheo, D. (2010). Biomarkers expression in rat olfactory ensheathing cells. Frontiers in Bioscience, 2, 289–298.
Mackay-Sim, A., & St. John, J. A. (2010). Olfactory ensheathing cells from the nose: clinical application in human spinal cord injuries. Experimental Neurology, 229, 174–180.
Rao, Y., Zhu, W., Liu, H., Jia, C., Zhao, Q., & Wang, Y. (2013). Clinical application of olfactory ensheathing cells in the treatment of spinal cord injury. Journal of International Medical Research, 41(2), 473–481.
Sethi, R., Sethi, R., Redmond, A., & Lavik, E. (2014). Olfactory ensheathing cells promote differentiation of neural stem cells and robust neurite extension. Stem Cell Reviews and Report, 10(6), 772–785.
Siddiqui, A. M., Khazaei, M., & Fehlings, M. G. (2015). Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Progress in Brain Research, 218, 15–54.
Guérout, N., Derambure, C., Drouot, L., Bon-Mardion, N., Duclos, C., Boyer, O., et al. (2010). Comparative gene expression profiling of olfactory ensheathing cells from olfactory bulb and olfactory mucosa. Glia, 58(13), 1570–1580.
Kueh, J. L., Raisman, G., Li, Y., Stevens, R., & Li, D. (2011). Comparison of bulbar and mucosal olfactory ensheathing cells using FACS and simultaneous antigenic bivariate cell cycle analysis. Glia, 59(11), 1658–1671.
Tabakow, P., Raisman, G., Fortuna, W., Czyz, M., Huberm, J., Li, D., et al. (2014). Functional regeneration of supraspinal connections in a patient with transected spinal cord following transplantation of bulbar olfactory ensheathing cells with peripheral nerve bridging. Cell Transplantation, 23(12), 1631–1655.
Yamamoto, M., Raisman, G., Li, D., & Li, Y. (2009). Transplanted olfactory mucosal cells restore paw reaching function without regeneration of severed corticospinal tract fibres across the lesion. Brain Research, 1303, 26–31.
Spees, J. L., Gregory, C. A., Singh, H., Tucker, H. A., Peister, A., Lynch, et al. (2004). Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Molecular Therapy, 9, 747–756.
Brindley, D. A., Davie, N. L., Culme-Seymour, E. J., Mason, C., Smith, D. W., & Rowley, J. A. (2012). Peak serum: implications of serum supply for cell therapy manufacturing. Regenerative Medicine, 7(1), 7–13.
Kuznetsov, S. A., Mankani, M. H., & Robey, P. G. (2000). Effect of serum on human bone marrow stromal cells: ex vivo expansion and in vivo bone formation. Transplantation, 70, 1780–1787.
Mark, P., Kleinsorge, M., Gaebel, R., Lux, C. A., Toelk, A., Pittermann, E., et al. (2013). Human mesenchymal stem cells display reduced expression of CD105 after culture in serum-free medium. Stem Cells International, 2013, 698076.
Hong, X., Chedid, K., & Kalkanis, S. N. (2012). Glioblastoma cell line-derived spheres in serum containing medium versus serum-free medium: a comparison of cancer stem cell properties. International Journal of Oncology, 41(5), 1693–1700.
Alexander, C. L., Fitzgerald, U. F., & Barnett, S. C. (2002). Identification of growth factors that promote long-term proliferation of olfactory ensheathing cells and modulate their antigenic phenotype. Glia, 37, 349–364.
Sharma, H. S. (2007). Neurotrophic factors in combination: a possible new therapeutic strategy to influence pathophysiology of spinal cord injury and repair mechanisms. Current Pharmaceutical Design, 13(18), 1841–1874.
Duan, D., & Lu, M. (2015). Olfactory mucosa: a rich source of cell therapy for central nervous system repair. Reviews in the Neurosciences, 26(3), 281–293.
Féron, F., Perry, C., Cochrane, J., Licina, P., Nowitzke, A., Urquhart, S., et al. (2005). Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain, 128, 2951–2960.
Huang, H., Chen, L., Wang, H., Xiu, B., Li, B., Wang, R., et al. (2003). Influence of patients' age on functional recovery after transplantation of olfactory ensheathing cells into injured spinal cord injury. Chinese Medical Journal, 116(10), 1488–1491.
Wu, J., Sun, T., Ye, C., Yao, J., Zhu, B., & He, H. (2012). Clinical observation of fetal olfactory ensheathing glia transplantation (OEGT) in patients with complete chronic spinal cord injury. Cell Transplantation, 21(Suppl 1), S33–S37.
Rosner, J., Avalos, P., Acosta, F., Liu, J., & Drazin, D. (2012). The potential for cellular therapy combined with growth factors in spinal cord injury. Stem Cells International, 2012, 826754.
Lo Furno, D., Pellitteri, R., Graziano, A., Giuffrida, R., Vancheri, C., Gili, E., et al. (2013). Differentiation of human adipose stem cells into neural phenotype by neuroblastoma-or olfactory ensheathing cells-conditioned medium. Journal of Cellular Physiology, 228, 2109–2118.
Shukla, S., Chaturvedi, R. K., Seth, K., Roy, N. S., & Agrawal, A. K. (2009). Enhanced survival and function of neural stem cells-derived dopaminergic neurons under influence of olfactory ensheathing cells in parkinsonian rats. Journal of Neurochemistry, 109, 436–451.
Shukla, A., Mohapatra, T. M., Parmar, D., & Seth, K. (2014). Neuroprotective potentials of neurotrophin rich olfactory ensheathing cells conditioned media against 6OHDA-induced oxidative damage. Free Radical Research, 48(5), 560–571.
Roet, K. C., Bossers, K., Franssen, E. H., Ruitenberg, M. J., & Verhaagen, J. (2011). A meta-analysis of microarray-based gene expression studies of olfactory bulb-derived olfactory ensheathing cells. Experimental Neurology, 229(1), 10–45.
Lakatos, A., Franklin, R. J. M., & Barnett, S. C. (2000). Olfactory ensheating cells and Schwann cells differ in their in vitro interactions with astrocytes. Glia, 32, 214–225.
Acknowledgments
We would like to thank Dr. Luigi Castoro for his useful technical help in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Suppl. Table 1
(DOC 38 kb)
Suppl. Table 2
(DOC 39 kb)
Suppl. Figure 1
Images by phase-contrast microscopy of representative fields of OECs grown in different conditions. On the left the figure shows OECs in serum (DMEM/FBS) and with GFs (bFGF or GDNF); on the right the figure shows OECs without serum (FBS-free DMEM) and with GFs (bFGF or GDNF). Scale bar 50 μm (GIF 1769 kb)
Suppl. Figure 2
Representative flow cytometry analysis of markers expression by OECs. (GIF 53 kb)
Suppl. Figure 3
Effect of serum depletion on cell proliferation. (A): Number of OECs recovered after one week of culture with or without 10 % serum addition in the medium by Trypan blue exclusion. (B): Proliferation of the OECs after one week of culture with or without 10 % serum addition in the medium evaluated by MTS assay. (GIF 131 kb)
Rights and permissions
About this article
Cite this article
Pellitteri, R., Cova, L., Zaccheo, D. et al. Phenotypic Modulation and Neuroprotective Effects of Olfactory Ensheathing Cells: a Promising Tool for Cell Therapy. Stem Cell Rev and Rep 12, 224–234 (2016). https://doi.org/10.1007/s12015-015-9635-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-015-9635-3