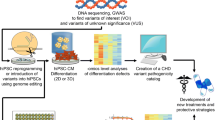
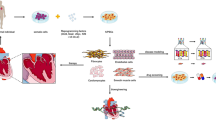
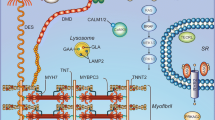
Abstract
Congenital heart disease (CHD) remains a significant health problem, with a growing population of survivors with chronic disease. Despite intense efforts to understand the genetic basis of CHD in humans, the etiology of most CHD is unknown. Furthermore, new models of CHD are required to better understand the development of CHD and to explore novel therapies for this patient population. In this review, we highlight the role that human induced pluripotent stem cell (hiPSC)-derived cardiomyocytes can serve to enhance our understanding of the development, pathophysiology and potential therapeutic targets for CHD. We highlight the use of hiPSC-derived cardiomyocytes to model gene regulatory interactions, cell-cell interactions and tissue interactions contributing to CHD. We further emphasize the importance of using hiPSC-derived cardiomyocytes as personalized research models. The use of hiPSCs presents an unprecedented opportunity to generate disease-specific cellular models, investigate the underlying molecular mechanisms of disease and uncover new therapeutic targets for CHD.





Similar content being viewed by others
References
Syrmou, A., Tzetis, M., Fryssira, H., Kosma, K., Oikonomakis, V., Giannikou, K., et al. (2013). Array comparative genomic hybridization as a clinical diagnostic tool in syndromic and nonsyndromic congenital heart disease. Pediatric Research, 73(6), 772–776.
Fahed, A. C., Gelb, B. D., Seidman, J. G., & Seidman, C. E. (2013). Genetics of congenital heart disease: the glass half empty. Circulation Research, 112(4), 707–720.
Pierpont, M. E., Basson, C. T., Benson, D. W., Gelb, B. D., Giglia, T. M., Goldmuntz, E., et al. (2007). Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American heart association congenital cardiac defects committee, council on cardiovascular disease in the young: endorsed by the American academy of pediatrics. Circulation, 115(23), 3015–3038.
Pueschel, S. M. (1990). Clinical aspects of Down syndrome from infancy to adulthood. American Journal of Medical Genetics Supplement, 752–756.
Goldhaber, S. Z., Brown, W. D., & Sutton, M. G. (1987). High frequency of mitral valve prolapse and aortic regurgitation among asymptomatic adults with Down’s syndrome. JAMA, 258(13), 1793–1795.
Freeman, S. B., Taft, L. F., Dooley, K. J., Allran, K., Sherman, S. L., Hassold, T. J., et al. (1998). Population-based study of congenital heart defects in Down syndrome. American Journal of Medical Genetics, 80(3), 213–217.
Källén, B., Mastroiacovo, P., & Robert, E. (1996). Major congenital malformations in Down syndrome. American Journal of Medical Genetics, 65(2), 160–166.
Prandstraller, D., Mazzanti, L., Picchio, F. M., Magnani, C., Bergamaschi, R., Perri, A., et al. (1999). Turner’s syndrome: cardiologic profile according to the different chromosomal patterns and long-term clinical follow-Up of 136 nonpreselected patients. Pediatric Cardiology, 20(2), 108–112.
Mazzanti, L., & Cacciari, E. (1998). Congenital heart disease in patients with Turner’s syndrome. Italian study group for turner syndrome (ISGTS). The Journal of Pediatrics, 133(5), 688–692.
Mazzanti, L., Prandstraller, D., Tassinari, D., Rubino, I., Santucci, S., Picchio, F. M., et al. (1988). Heart disease in Turner’s syndrome. Helvetica Paediatrica Acta, 43(1–2), 25–31.
Lin, A. E., Lippe, B. M., Geffner, M. E., Gomes, A., Lois, J. F., Barton, C. W., et al. (1986). Aortic dilation, dissection, and rupture in patients with Turner syndrome. The Journal of Pediatrics, 109(5), 820–826.
Lin, A. E., Lippe, B., & Rosenfeld, R. G. (1998). Further delineation of aortic dilation, dissection, and rupture in patients with Turner syndrome. Pediatrics, 102(1), e12.
Van Praagh, S., Truman, T., Firpo, A., Bano-Rodrigo, A., Fried, R., McManus, B., et al. (1989). Cardiac malformations in trisomy-18: a study of 41 postmortem cases. Journal of the American College of Cardiology, 13(7), 1586–1597.
Matsuoka, R., Misugi, K., Goto, A., Gilbert, E. F., & Ando, M. (1983). Congenital heart anomalies in the trisomy 18 syndrome, with reference to congenital polyvalvular disease. American Journal of Medical Genetics, 14(4), 657–668.
Ryan, A. K., Goodship, J. A., Wilson, D. I., Philip, N., Levy, A., Seidel, H., et al. (1997). Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. Journal of Medical Genetics, 34(10), 798–804.
McDonald-McGinn, D. M., Kirschner, R., Goldmuntz, E., Sullivan, K., Eicher, P., Gerdes, M., et al. (1999). The Philadelphia story: the 22q11.2 deletion: report on 250 patients. Genetic Counseling, 10(1), 11–24.
Johnson, T. R., Goldmuntz, E., McDonald-McGinn, D. M., Zackai, E. H., & Fogel, M. A. (2005). Cardiac magnetic resonance imaging for accurate diagnosis of aortic arch anomalies in patients with 22q11.2 deletion. The American Journal of Cardiology, 96(12), 1726–1730.
McDonald-McGinn, D. M., LaRossa, D., Goldmuntz, E., Sullivan, K., Eicher, P., Gerdes, M., et al. (1997). The 22q11.2 deletion: screening, diagnostic workup, and outcome of results; report on 181 patients. Genetic Testing, 1(2), 99–108.
Eronen, M., Peippo, M., Hiippala, A., Raatikka, M., Arvio, M., Johansson, R., & Kähkönen, M. (2002). Cardiovascular manifestations in 75 patients with Williams syndrome. Journal of Medical Genetics, 39(8), 554–558.
Bruno, E., Rossi, N., Thüer, O., Córdoba, R., & Alday, L. E. (2003). Cardiovascular findings, and clinical course, in patients with Williams syndrome. Cardiology in the Young, 13(6), 532–536.
Wu, Y. Q., Sutton, V. R., Nickerson, E., Lupski, J. R., Potocki, L., Korenberg, J. R., et al. (1998). Delineation of the common critical region in Williams syndrome and clinical correlation of growth, heart defects, ethnicity, and parental origin. American Journal of Medical Genetics, 78(1), 82–89.
Grossfeld, P. D., Mattina, T., Lai, Z., Favier, R., Jones, K. L., Cotter, F., & Jones, C. (2004). The 11q terminal deletion disorder: a prospective study of 110 cases. American Journal of Medical Genetics Part A, 129A(1), 51–61.
Tartaglia, M., Kalidas, K., Shaw, A., Song, X., Musat, D. L., van der Burgt, I., et al. (2002). PTPN11 mutations in Noonan syndrome: molecular spectrum, genotype-phenotype correlation, and phenotypic heterogeneity. The American Journal of Human Genetics, 70(6), 1555–1563.
Tartaglia, M., Mehler, E. L., Goldberg, R., Zampino, G., Brunner, H. G., Kremer, H., et al. (2001). Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nature Genetics, 29(4), 465–468.
Tartaglia, M., Pennacchio, L. A., Zhao, C., Yadav, K. K., Fodale, V., Sarkozy, A., et al. (2007). Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nature Genetics, 39(1), 75–79.
Pandit, B., Sarkozy, A., Pennacchio, L. A., Carta, C., Oishi, K., Martinelli, S., et al. (2007). Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nature Genetics, 39(8), 1007–1012.
Sarkozy, A., Carta, C., Moretti, S., Zampino, G., Digilio, M. C., Pantaleoni, F., et al. (2009). Germline BRAF mutations in Noonan, LEOPARD, and cardiofaciocutaneous syndromes: molecular diversity and associated phenotypic spectrum. Human Mutation, 30(4), 695–702.
Bertola, D. R., Yamamoto, G. L., Almeida, T. F., Buscarilli, M., Jorge, A. A., Malaquias, A. C., et al. (2014). Further evidence of the importance of RIT1 in Noonan syndrome. American Journal of Medical Genetics Part A, 164A(11), 2952–2957.
Li, L., Krantz, I. D., Deng, Y., Genin, A., Banta, A. B., Collins, C. C., et al. (1997). Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nature Genetics, 16(3), 243–251.
McElhinney, D. B., Krantz, I. D., Bason, L., Piccoli, D. A., Emerick, K. M., Spinner, N. B., & Goldmuntz, E. (2002). Analysis of cardiovascular phenotype and genotype-phenotype correlation in individuals with a JAG1 mutation and/or Alagille syndrome. Circulation, 106(20), 2567–2574.
Ruiz-Perez, V. L., Ide, S. E., Strom, T. M., Lorenz, B., Wilson, D., Woods, K., et al. (2000). Mutations in a new gene in Ellis-van Creveld syndrome and Weyers acrodental dysostosis. Nature Genetics, 24(3), 283–286.
Ruiz-Perez, V. L., Tompson, S. W., Blair, H. J., Espinoza-Valdez, C., Lapunzina, P., Silva, E. O., et al. (2003). Mutations in two nonhomologous genes in a head-to-head configuration cause Ellis-van Creveld syndrome. The American Journal of Human Genetics, 72(3), 728–732.
Basson, C. T., Bachinsky, D. R., Lin, R. C., Levi, T., Elkins, J. A., Soults, J., et al. (1997). Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nature Genetics, 15(1), 30–35.
Basson, C. T., Huang, T., Lin, R. C., Bachinsky, D. R., Weremowicz, S., Vaglio, A., et al. (1999). Different TBX5 interactions in heart and limb defined by Holt-Oram syndrome mutations. Proceedings of the National Academy of Sciences of the United States of America, 96(6), 2919–2924.
Ferencz, C., Boughman, J. A., Neill, C. A., Brenner, J. I., & Perry, L. W. (1989). Congenital cardiovascular malformations: questions on inheritance. Baltimore-Washington infant study group. Journal of the American College of Cardiology, 14(3), 756–763.
(2010). Heart development and regeneration set [Internet]. Amsterdam: Academic Press. Available from: http://www.sciencedirect.com/science/book/9780123813329.
Martinsen, B., Lohr, J. L. (2009). Cardiac Development. In Handbook of Cardiac Anatomy, Physiology, and Devices.
Bruneau, B. G. (2013). Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harbor Perspectives in Biology, 5(3), a008292.
Buckingham, M., Meilhac, S., & Zaffran, S. (2005). Building the mammalian heart from two sources of myocardial cells. Nature Reviews Genetics, 6(11), 826–835.
Brade, T., Pane, L. S., Moretti, A., Chien, K. R., & Laugwitz, K. L. (2013). Embryonic heart progenitors and cardiogenesis. Cold Spring Harbor Perspective Medicine, 3(10), a013847.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872.
Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318(5858), 1917–1920.
Takahashi, K., & Yamanaka, S. (2013). Induced pluripotent stem cells in medicine and biology. Development, 140(12), 2457–2461.
Matsa, E., Burridge, P. W., & Wu, J. C. (2014). Human stem cells for modeling heart disease and for drug discovery. Science Translational Medicine, 6(239), 239ps6.
Burridge, P. W., Keller, G., Gold, J. D., & Wu, J. C. (2012). Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell, 10(1), 16–28.
Mummery, C. L., Zhang, J., Ng, E. S., Elliott, D. A., Elefanty, A. G., & Kamp, T. J. (2012). Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circulation Research, 111(3), 344–358.
Burridge, P. W., Matsa, E., Shukla, P., Lin, Z. C., Churko, J. M., Ebert, A. D., et al. (2014). Chemically defined generation of human cardiomyocytes. Nature Methods, 11(8), 855–860.
Graichen, R., Xu, X., Braam, S. R., Balakrishnan, T., Norfiza, S., Sieh, S., et al. (2008). Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation, 76(4), 357–370.
Jang, J., Ku, S. Y., Kim, J. E., Choi, K., Kim, Y. Y., Kim, H. S., et al. (2008). Notch inhibition promotes human embryonic stem cell-derived cardiac mesoderm differentiation. Stem Cells, 26 (11), 2782–2790.
Yu, X., Zou, J., Ye, Z., Hammond, H., Chen, G., Tokunaga, A., et al. (2008). Notch signaling activation in human embryonic stem cells is required for embryonic, but not trophoblastic, lineage commitment. Cell Stem Cell, 2(5), 461–471.
Kattman, S. J., Witty, A. D., Gagliardi, M., Dubois, N. C., Niapour, M., Hotta, A., et al. (2011). Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell, 8(2), 228–240.
Lian, X., Hsiao, C., Wilson, G., Zhu, K., Hazeltine, L. B., Azarin, S. M., et al. (2012). Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America, 109(27), E1848–E1857.
Yang, L., Soonpaa, M. H., Adler, E. D., Roepke, T. K., Kattman, S. J., Kennedy, M., et al. (2008). Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature, 453(7194), 524–528.
Ema, M., Takahashi, S., & Rossant, J. (2006). Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood, 107(1), 111–117.
Dunn, N. R., Vincent, S. D., Oxburgh, L., Robertson, E. J., & Bikoff, E. K. (2004). Combinatorial activities of Smad2 and Smad3 regulate mesoderm formation and patterning in the mouse embryo. Development, 131(8), 1717–1728.
Laflamme, M. A., Chen, K. Y., Naumova, A. V., Muskheli, V., Fugate, J. A., Dupras, S. K., et al. (2007). Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotechnology, 25(9), 1015–1024.
Zhang, J., Klos, M., Wilson, G. F., Herman, A. M., Lian, X., Raval, K. K., et al. (2012). Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circulation Research, 111(9), 1125–1136.
Cai, W., Guzzo, R. M., Wei, K., Willems, E., & Mercola, M. (2012). A Nodal-to-TGFβ cascade exerts biphasic control over cardiopoiesis. Circluation Research, 111(7), 876–881.
Tran, T. H., Wang, X., Browne, C., Zhang, Y., Schinke, M., Izumo, S., & Burcin, M. (2009). Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells, 27(8), 1869–1878.
Paige, S. L., Osugi, T., Afanasiev, O. K., Pabon, L., Reinecke, H., & Murry, C. E. (2010). Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PloS One, 5(6), e11134.
Snir, M., Kehat, I., Gepstein, A., Coleman, R., Itskovitz-Eldor, J., Livne, E., & Gepstein, L. (2003). Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. American Journal of Physiology - Heart and Circulatory Physiology, 285(6), H2355–H2363.
Lundy, S. D., Zhu, W. Z., Regnier, M., & Laflamme, M. A. (2013). Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells and Development, 22(14), 1991–2002.
Bedada, F. B., Chan, S. S., Metzger, S. K., Zhang, L., Zhang, J., Garry, D. J., et al. (2014). Acquisition of a quantitative, stoichiometrically conserved ratiometric marker of maturation status in stem cell-derived cardiac myocytes. Stem Cell Reports, 3(4), 594–605.
Willems, E., Spiering, S., Davidovics, H., Lanier, M., Xia, Z., Dawson, M., et al. (2011). Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circulation Research, 109(4), 360–364.
Hudson, J. E., & Zimmermann, W. H. (2011). Tuning Wnt-signaling to enhance cardiomyogenesis in human embryonic and induced pluripotent stem cells. Journal of Molecular and Cellular Cardiology, 51(3), 277–279.
Ren, Y., Lee, M. Y., Schliffke, S., Paavola, J., Amos, P. J., Ge, X., et al. (2011). Small molecule Wnt inhibitors enhance the efficiency of BMP-4-directed cardiac differentiation of human pluripotent stem cells. Journal of Molecular and Cellular Cardiology, 51(3), 280–287.
Karakikes, I., Senyei, G. D., Hansen, J., Kong, C. W., Azeloglu, E. U., Stillitano, F., et al. (2014). Small molecule-mediated directed differentiation of human embryonic stem cells toward ventricular cardiomyocytes. Stem Cells Translational Medicine, 3(1), 18–31.
Witty, A. D., Mihic, A., Tam, R. Y., Fisher, S. A., Mikryukov, A., Shoichet, M. S., et al. (2014). Generation of the epicardial lineage from human pluripotent stem cells. Nature Biotechnology, 32(10), 1026–1035.
Später, D., Abramczuk, M. K., Buac, K., Zangi, L., Stachel, M. W., Clarke, J., et al. (2013). A HCN4+ cardiomyogenic progenitor derived from the first heart field and human pluripotent stem cells. Nature Cell Biology, 15(9), 1098–1106.
Elliott, D. A., Braam, S. R., Koutsis, K., Ng, E. S., Jenny, R., Lagerqvist, E. L., et al. (2011). NKX2-5(eGFP/w) hESCs for isolation of human cardiac progenitors and cardiomyocytes. Nature Methods, 8(12), 1037–1040.
Bruneau, B. G., Nemer, G., Schmitt, J. P., Charron, F., Robitaille, L., Caron, S., et al. (2001). A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell, 106(6), 709–721.
Takeuchi, J. K., Ohgi, M., Koshiba-Takeuchi, K., Shiratori, H., Sakaki, I., Ogura, K., et al. (2003). Tbx5 specifies the left/right ventricles and ventricular septum position during cardiogenesis. Development, 130(24), 5953–5964.
Liang, X., Wang, G., Lin, L., Lowe, J., Zhang, Q., Bu, L., et al. (2013). HCN4 dynamically marks the first heart field and conduction system precursors. Circulation Research, 113(4), 399–407.
Bu, L., Jiang, X., Martin-Puig, S., Caron, L., Zhu, S., Shao, Y., et al. (2009). Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature, 460(7251), 113–117.
Moretti, A., Caron, L., Nakano, A., Lam, J. T., Bernshausen, A., Chen, Y., et al. (2006). Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell, 127(6), 1151–1165.
Buikema, J. W., Zwetsloot, P. P., Doevendans, P. A., Sluijter, J. P., & Domian, I. J. (2013). Expanding mouse ventricular cardiomyocytes through GSK-3 inhibition. Current Protocols in Cell Biology, 61, 23.9.1–23.9.10.
Cao, N., Liang, H., Huang, J., Wang, J., Chen, Y., Chen, Z., and Yang, H. T. (2013). Highly efficient induction and long-term maintenance of multipotent cardiovascular progenitors from human pluripotent stem cells under defined conditions. Cell Research, 23(9), 1119–1132.
Lian, X., Bao, X., Al-Ahmad, A., Liu, J., Wu, Y., Dong, W., et al. (2014). Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling. Stem Cell Reports, 3(5), 804–816.
He, J. Q., Ma, Y., Lee, Y., Thomson, J. A., & Kamp, T. J. (2003). Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circulation Research, 93(1), 32–39.
Xu, C., Police, S., Rao, N., & Carpenter, M. K. (2002). Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circulation Research, 91(6), 501–508.
Tanwar, V., Bylund, J. B., Hu, J., Yan, J., Walthall, J. M., Mukherjee, A., et al. (2014). Gremlin 2 promotes differentiation of embryonic stem cells to atrial fate by activation of the JNK signaling pathway. Stem Cells, 32(7), 1774–1788.
Zhang, Q., Jiang, J., Han, P., Yuan, Q., Zhang, J., Zhang, X., et al. (2011). Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Research, 21(4), 579–587.
Caspi, O., Huber, I., Kehat, I., Habib, M., Arbel, G., Gepstein, A., et al. (2007). Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. Journal of the American College of Cardiology, 50(19), 1884–1893.
van Laake, L. W., Passier, R., Monshouwer-Kloots, J., Nederhoff, M. G., Ward-van Oostwaard, D., Field, L. J., et al. (2007). Monitoring of cell therapy and assessment of cardiac function using magnetic resonance imaging in a mouse model of myocardial infarction. Nature Protocols, 2(10), 2551–2567.
Laflamme, M. A., Gold, J., Xu, C., Hassanipour, M., Rosler, E., Police, S., et al. (2005). Formation of human myocardium in the rat heart from human embryonic stem cells. The American Journal of Pathology, 167(3), 663–671.
Fernandes, S., Naumova, A. V., Zhu, W. Z., Laflamme, M. A., Gold, J., & Murry, C. E. (2010). Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. Journal of Molecular and Cellular Cardiology, 49(6), 941–949.
Shiba, Y., Fernandes, S., Zhu, W. Z., Filice, D., Muskheli, V., Kim, J., et al. (2012). Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature, 489(7415), 322–325.
Chong, J. J., Yang, X., Don, C. W., Minami, E., Liu, Y. W., Weyers, J. J., et al. (2014). Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature, 510(7504), 273–277.
Yang, X., Pabon, L., & Murry, C. E. (2014). Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circulation Research, 114(3), 511–523.
Kamakura, T., Makiyama, T., Sasaki, K., Yoshida, Y., Wuriyanghai, Y., Chen, J., et al. (2013). Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circulation Journal, 77(5), 1307–1314.
Ivashchenko, C. Y., Pipes, G. C., Lozinskaya, I. M., Lin, Z., Xu, X., Needle, S., et al. (2013). Human induced pluripotent stem cell derived cardiomyocytes exhibit temporal changes in phenotype. American Journal Physiology Heart and Circulatory Physiology, 305(6), H913–922.
Kobayashi, J., Yoshida, M., Tarui, S., Hirata, M., Nagai, Y., Kasahara, S., et al. (2014). Directed differentiation of patient-specific induced pluripotent stem cells identifies the transcriptional repression and epigenetic modification of NKX2-5, HAND1, and NOTCH1 in hypoplastic left heart syndrome. PloS One, 9(7), e102796.
Jiang, Y., Habibollah, S., Tilgner, K., Collin, J., Barta, T., Al-Aama, J. Y., et al. (2014). An induced pluripotent stem cell model of hypoplastic left heart syndrome (HLHS) reveals multiple expression and functional differences in HLHS-derived cardiac myocytes. Stem Cells Translational Medicine, 3(4), 416–423.
Letourneau, A., Santoni, F. A., Bonilla, X., Sailani, M. R., Gonzalez, D., Kind, J., et al. (2014). Domains of genome-wide gene expression dysregulation in Down’s syndrome. Nature, 508(7496), 345–350.
Bosman, A., Letourneau, A., Sartiani, L., Del Lungo, M., Ronzoni, F., Kuziakiv, R., et al. (2015). Perturbations of heart development and function in cardiomyocytes from hESC with trisomy 21. Stem Cells, 33(5), 1434–1446.
Carvajal-Vergara, X., Sevilla, A., D’Souza, S. L., Ang, Y. S., Schaniel, C., Lee, D. F., et al. (2010). Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature, 465(7299), 808–812.
Kinnear, C., Chang, W. Y., Khattak, S., Hinek, A., Thompson, T., de Carvalho Rodrigues, D., et al. (2013). Modeling and rescue of the vascular phenotype of Williams-Beuren syndrome in patient induced pluripotent stem cells. Stem Cells Translational Medicine, 2(1), 2–15.
Ge, X., Ren, Y., Bartulos, O., Lee, M. Y., Yue, Z., Kim, K. Y., et al. (2012). Modeling supravalvular aortic stenosis syndrome with human induced pluripotent stem cells. Circulation, 126(14), 1695–1704.
Sun, N., Yazawa, M., Liu, J., Han, L., Sanchez-Freire, V., Abilez, O. J., et al. (2012). Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Science Translational Medicine, 4(130), 130ra47.
Siu, C. W., Lee, Y. K., Ho, J. C., Lai, W. H., Chan, Y. C., Ng, K. M., et al. (2012). Modeling of lamin A/C mutation premature cardiac aging using patient‐specific induced pluripotent stem cells. Aging (Albany NY), 4(11), 803–822.
Tse, H. F., Ho, J. C., Choi, S. W., Lee, Y. K., Butler, A. W., Ng, K. M., et al. (2013). Patient-specific induced-pluripotent stem cells-derived cardiomyocytes recapitulate the pathogenic phenotypes of dilated cardiomyopathy due to a novel DES mutation identified by whole exome sequencing. Human Molecular Genetics, 22(7), 1395–1403.
Lan, F., Lee, A. S., Liang, P., Sanchez-Freire, V., Nguyen, P. K., Wang, L., et al. (2013). Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell, 12(1), 101–113.
Han, L., Li, Y., Tchao, J., Kaplan, A. D., Lin, B., Li, Y., et al. (2014). Study familial hypertrophic cardiomyopathy using patient-specific induced pluripotent stem cells. Cardiovascular Research, 104(2), 258–269.
Egashira, T., Yuasa, S., Suzuki, T., Aizawa, Y., Yamakawa, H., Matsuhashi, T., et al. (2012). Disease characterization using LQTS-specific induced pluripotent stem cells. Cardiovascular Research, 95(4), 419–429.
Moretti, A., Bellin, M., Welling, A., Jung, C. B., Lam, J. T., Bott-Flügel, L., et al. (2010). Patient-specific induced pluripotent stem-cell models for long-QT syndrome. The New England Journal of Medicine, 363(15), 1397–1409.
Matsa, E., Rajamohan, D., Dick, E., Young, L., Mellor, I., Staniforth, A., & Denning, C. (2011). Drug evaluation in cardiomyocytes derived from human induced pluripotent stem cells carrying a long QT syndrome type 2 mutation. European Heart Journal, 32(8), 952–962.
Itzhaki, I., Maizels, L., Huber, I., Zwi-Dantsis, L., Caspi, O., Winterstern, A., et al. (2011) Modelling the long QT syndrome with induced pluripotent stem cells. Nature, 471(7337), 225–229.
Ma, D., Wei, H., Zhao, Y., Lu, J., Li, G., Sahib, N. B., et al. (2013). Modeling type 3 long QT syndrome with cardiomyocytes derived from patient-specific induced pluripotent stem cells. International Journal of Cardiology, 168(6), 5277–5286.
Terrenoire, C., Wang, K., Tung, K. W., Chung, W. K., Pass, R. H., Lu, J. T., et al. (2013). Induced pluripotent stem cells used to reveal drug actions in a long QT syndrome family with complex genetics. The Journal of General Physiology, 141(1), 61–72.
Yazawa, M., Hsueh, B., Jia, X., Pasca, A. M., Bernstein, J. A., Hallmayer, J., & Dolmetsch, R. E. (2011). Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature, 471(7337), 230–234.
Itzhaki, I., Maizels, L., Huber, I., Gepstein, A., Arbel, G., Caspi, O., et al. (2012). Modeling of catecholaminergic polymorphic ventricular tachycardia with patient-specific human-induced pluripotent stem cells. Journal of the American College of Cardiology, 60(11):990–1000.
Ma, D., Wei, H., Lu, J., Ho, S., Zhang, G., Sun, X., et al. (2013). Generation of patient-specific induced pluripotent stem cellderived cardiomyocytes as a cellular model of arrhythmogenic right ventricular cardiomyopathy. European Heart Journal, 34(15):1122–33.
Kim, C., Wong, J., Wen, J., Wang, S., Wang, C., Spiering, S., et al. (2013). Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs. Nature, 494(7435), 105–110.
Wang, G., McCain, M. L., Yang, L., He, A., Pasqualini, F. S., Agarwal, A., et al. (2014). Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nature Medicine, 20(6), 616–623.
Huang, H. P., Chen, P. H., Hwu, W. L., Chuang, C. Y., Chien, Y. H., Stone, L., et al. (2011). Human Pompe disease-induced pluripotent stem cells for pathogenesis modeling, drug testing and disease marker identification. Human Molecular Genetics, 20(24), 4851–4864.
Raval, K. K., Tao, R., White, B. E., De Lange, W. J., Koonce, C. H., Yu, J., et al. (2014) Pompe disease results in a Golgi-based glycosylation deficit in human induced pluripotent stem cell-derived cardiomyocytes. The Journal of Biological Chemistry, 290(5), 3121-3136.
Liu, J., Verma, P. J., Evans-Galea, M. V., Delatycki, M. B., Michalska, A., Leung, J., et al. (2011). Generation of induced pluripotent stem cell lines from Friedreich ataxia patients. Stem Cell Reviews, 7(3), 703–713.
Lee, Y. K., Ho, P. W., Schick, R., Lau, Y. M., Lai, W. H., Zhou, T., et al. (2014). Modeling of Friedreich ataxia-related iron overloading cardiomyopathy using patient-specific-induced pluripotent stem cells. Pflügers Archiv, 466(9), 1831–1844.
Hick, A., Wattenhofer-Donzé, M., Chintawar, S., Tropel, P., Simard, J. P., Vaucamps, N., et al. (2013). Neurons and cardiomyocytes derived from induced pluripotent stem cells as a model for mitochondrial defects in Friedreich’s ataxia. Disease Models & Mechanisms, 6(3), 608–621.
Sallam, K., Kodo, K., & Wu, J. C. (2014). Modeling inherited cardiac disorders. Circulation Journal, 78(4), 784–794.
Yazawa, M., & Dolmetsch, R. E. (2013). Modeling Timothy syndrome with iPS cells. Journal of Cardiovascular Translational Research, 6(1), 1–9.
Matsa, E., Dixon, J. E., Medway, C., Georgiou, O., Patel, M. J., Morgan, K., et al. (2014). Allele-specific RNA interference rescues the long-QT syndrome phenotype in human-induced pluripotency stem cell cardiomyocytes. European Heart Journal, 35(16), 1078–1087.
Okata, S., Yuasa, S., Yamane, T., Furukawa, T., & Fukuda, K. (2013). The generation of induced pluripotent stem cells from a patient with KCNH2 G603D, without LQT2 disease associated symptom. Journal of Medical and Dental Sciences, 60(1), 17–22.
Fatima, A., Kaifeng, S., Dittmann, S., Xu, G., Gupta, M. K., Linke, M., et al. (2013). The disease-specific phenotype in cardiomyocytes derived from induced pluripotent stem cells of two long QT syndrome type 3 patients. PloS One, 8(12), e83005.
Mehta, A., Sequiera, G. L., Ramachandra, C. J., Sudibyo, Y., Chung, Y., Sheng, J., et al. (2014). Re-trafficking of hERG reverses long QT syndrome 2 phenotype in human iPS-derived cardiomyocytes. Cardiovascular Research, 102(3), 497–506.
Bellin, M., Casini, S., Davis, R. P., D’Aniello, C., Haas, J., Ward-van Oostwaard, D., et al. (2013). Isogenic human pluripotent stem cell pairs reveal the role of a KCNH2 mutation in long-QT syndrome. The EMBO Journal, 32(24), 3161–3175.
Malan, D., Friedrichs, S., Fleischmann, B. K., & Sasse, P. (2011). Cardiomyocytes obtained from induced pluripotent stem cells with long-QT syndrome 3 recapitulate typical disease-specific features in vitro. Circulation Research, 109(8), 841–847.
Eschenhagen, T., Mummery, C., & Knollmann, B. C. (2015). Modelling sarcomeric cardiomyopathies in the dish: from human heart samples to iPSC cardiomyocytes. Cardiovascular Research, 105(4), 424–438.
Josowitz, R., Carvajal-Vergara, X., Lemischka, I. R., & Gelb, B. D. (2011). Induced pluripotent stem cell-derived cardiomyocytes as models for genetic cardiovascular disorders. Current Opinion in Cardiology, 26(3), 223–229.
Gaber, N., Gagliardi, M., Patel, P., Kinnear, C., Zhang, C., Chitayat, D., et al. (2013). Fetal reprogramming and senescence in hypoplastic left heart syndrome and in human pluripotent stem cells during cardiac differentiation. The American Journal of Pathology, 183(3), 720–734.
Schachterle, W., Rojas, A., Xu, S. M., & Black, B. L. (2012). ETS-dependent regulation of a distal Gata4 cardiac enhancer. Developmental Biology, 361(2), 439–449.
Majka, S. M., & McGuire, P. G. (1997). Regulation of urokinase expression in the developing avian heart: a role for the Ets-2 transcription factor. Mechanisms of Development, 68(1–2), 127–137.
Wamstad, J. A., Alexander, J. M., Truty, R. M., Shrikumar, A., Li, F., Eilertson, K. E., et al. (2012). Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell, 151(1), 206–220.
Paige, S. L., Thomas, S., Stoick-Cooper, C. L., Wang, H., Maves, L., Sandstrom, R., et al. (2012). A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell, 151(1), 221–232.
Zaidi, S., Choi, M., Wakimoto, H., Ma, L., Jiang, J., Overton, J. D., et al. (2013). De novo mutations in histone-modifying genes in congenital heart disease. Nature, 498(7453), 220–223.
Hiroi, Y., Kudoh, S., Monzen, K., Ikeda, Y., Yazaki, Y., Nagai, R., & Komuro, I. (2001). Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation. Nature Genetics, 28(3), 276–280.
Ghosh, T. K., Song, F. F., Packham, E. A., Buxton, S., Robinson, T. E., Ronksley, J., et al. (2009). Physical interaction between TBX5 and MEF2C is required for early heart development. Molecular and Cellular Biology, 29(8), 2205–2218.
Kathiresan, S., & Srivastava, D. (2012). Genetics of human cardiovascular disease. Cell, 148(6), 1242–1257.
Bruneau, B. G. (2008). The developmental genetics of congenital heart disease. Nature, 451(7181), 943–948.
Garg, V., Kathiriya, I. S., Barnes, R., Schluterman, M. K., King, I. N., Butler, C. A., et al. (2003). GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature, 424(6947), 443–447.
Pu, W. T., Ishiwata, T., Juraszek, A. L., Ma, Q., & Izumo, S. (2004). GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Developmental Biology, 275(1), 235–244.
Rajagopal, S. K., Ma, Q., Obler, D., Shen, J., Manichaikul, A., Tomita-Mitchell, A., et al. (2007). Spectrum of heart disease associated with murine and human GATA4 mutation. Journal of Molecular and Cellular Cardiology, 43(6), 677–685.
Treutlein, B., Brownfield, D. G., Wu, A. R., Neff, N. F., Mantalas, G. L., Espinoza, F. H., et al. (2014). Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature, 509(7500), 371–375.
Neph, S., Stergachis, A. B., Reynolds, A., Sandstrom, R., Borenstein, E., & Stamatoyannopoulos, J. A. (2012). Circuitry and dynamics of human transcription factor regulatory networks. Cell, 150(6), 1274–1286.
Gong, W., Koyano-Nakagawa, N., Li, T., Garry, D. J. (2015) Inferring dynamic gene regulatory networks in cardiac differentiation through the integration of multi-dimensional data. BMC Bioinformatics, 16(1), 74.
Lim, J., & Thiery, J. P. (2012). Epithelial-mesenchymal transitions: insights from development. Development, 139(19), 3471–3486.
Garg, V., Muth, A. N., Ransom, J. F., Schluterman, M. K., Barnes, R., King, I. N., et al. (2005). Mutations in NOTCH1 cause aortic valve disease. Nature, 437(7056), 270–274.
Fischer, A., Steidl, C., Wagner, T. U., Lang, E., Jakob, P. M., Friedl, P., et al. (2007). Combined loss of Hey1 and HeyL causes congenital heart defects because of impaired epithelial to mesenchymal transition. Circulation Research, 100(6), 856–863.
Greenway, S. C., Pereira, A. C., Lin, J. C., DePalma, S. R., Israel, S. J., Mesquita, S. M., et al. (2009). De novo copy number variants identify new genes and loci in isolated sporadic tetralogy of Fallot. Nature Genetics, 41(8), 931–935.
Ott, H. C., Matthiesen, T. S., Goh, S. K., Black, L. D., Kren, S. M., Netoff, T. I., & Taylor, D. A. (2008). Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nature Medicine, 14(2), 213–221.
Lu, T. Y., Lin, B., Kim, J., Sullivan, M., Tobita, K., Salama, G., Yang, L. (2013). Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nature Communications, 42307.
Grosberg, A., Alford, P. W., McCain, M. L., & Parker, K. K. (2011). Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab on a Chip, 11(24), 4165–4173.
Feinberg, A. W., Feigel, A., Shevkoplyas, S. S., Sheehy, S., Whitesides, G. M., & Parker, K. K. (2007). Muscular thin films for building actuators and powering devices. Science, 317(5843), 1366–1370.
Acknowledgments
We thank Cynthia DeKay and Erik Munsen for assistance with the graphic illustrations. This work was supported by funding from the NHLBI (1R01HL122576) and NHLBI Progenitor Cell Biology Consortium (5U01HL100407).
Conflicts of interest
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doyle, M.J., Lohr, J.L., Chapman, C.S. et al. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes as a Model for Heart Development and Congenital Heart Disease. Stem Cell Rev and Rep 11, 710–727 (2015). https://doi.org/10.1007/s12015-015-9596-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-015-9596-6