Abstract
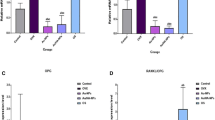
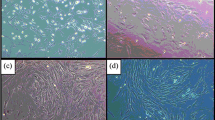
We investigated deleterious changes that take place in mesenchymal stem cells (MSC) and its fracture healing competence in ovariectomy (Ovx)-induced osteopenia. MSC from bone marrow (BM) of ovary intact (control) and Ovx rats was isolated. 99mTc-HMPAO (Technitium hexamethylpropylene amine oxime) labeled MSC was systemically transplanted to rats and fracture tropism assessed by SPECT/CT. PKH26 labeled MSC (PKH26-MSC) was bound in scaffold and applied to fracture site (drill-hole in femur metaphysis). Osteoinduction was quantified by calcein binding and microcomputed tomography. Estrogen receptor (ER) antagonist, fulvestrant was used to determine ER dependence of osteo-induction by MSC. BM-MSC number was strikingly reduced and doubling time increased in Ovx rats compared to control. SPECT/CT showed reduced localization of 99mTc-HMPAO labeled MSC to the fracture site, 3 h post-transplantation in Ovx rats as compared with controls. Post-transplantation, Ovx MSC labeled with PKH26 (Ovx PKH26-MSC) localized less to fracture site than control PKH26-MSC. Transplantation of either control or Ovx MSC enhanced calcein binding and bone volume at the callus of control rats over placebo group however Ovx MSC had lower efficacy than control MSC. Fulvestrant blocked osteoinduction by control MSC. When scaffold bound MSC was applied to fracture, osteoinduction by Ovx PKH26-MSC was less than control PKH26-MSC. In Ovx rats, control MSC/E2 treatment but not Ovx MSC showed osteoinduction. Regenerated bone was irregularly deposited in Ovx MSC group. In conclusion, Ovx is associated with diminished BM-MSC number and its growth, and Ovx MSC displays impaired engraftment to fracture and osteoinduction besides disordered bone regeneration.







Similar content being viewed by others
References
Gruber, R., Koch, H., Doll, B. A., Tegtmeier, F., Einhorn, T. A., & Hollinger, J. O. (2006). Fracture healing in the elderly patient. Experimental Gerontology, 41(11), 1080–1093.
Elford K., & Claman P., (2004). Osteoporosis in reproductive age women: Impact of sex steroid replacement. Encyclopedia of Endocrine Diseases, pp. 441–446
Balasch, J. (2003). Sex steroids and bone: current perspectives. Human Reproduction Update, 9(3), 207–222.
Bethel, M., Chitteti, B. R., Srour, E. F., & Kacena, M. A. (2013). The changing balance between osteoblastogenesis and adipogenesis in aging and its impact on hematopoiesis. Current Osteoporosis Reports, 11(2), 99–106.
Arrabal, P. M., Visser, R., Santos-Ruiz, L., Becerra, J., & Cifuentes, M. (2013). Osteogenic molecules for clinical applications: improving the BMP-collagen system. Biological Research, 46(4), 421–429.
Nevins, M. L., & Reynolds, M. A. (2011). Tissue engineering with recombinant human platelet-derived growth factor BB for implant site development. Compendium of Continuing Education in Dentistry, 32(2), 18. 20–7.
Oryan, A., Alidadi, S., Moshiri, A., & Maffulli, N. (2014). Bone regenerative medicine: classic options, novel strategies, and future directions. Journal of Orthopaedic Surgery and Research, 9(1), 18. doi:10.1186/1749-799X-9-18.
Liebergall, M., Schroeder, J., Mosheiff, R., et al. (2013). Stem cell-based therapy for prevention of delayed fracture union: a randomized and prospective preliminary study. Molecular Therapy, 21(8), 1631–1638.
Frohlich, M., Grayson, W. L., Wan, L. Q., Marolt, D., Drobnic, M., & Vunjak-Novakovic, G. (2008). Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Current Stem Cell Research & Therapy, 3(4), 254–264.
Kean, T. J., Lin, P., Caplan, A. I., & Dennis, J. E. (2013). MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells International. doi:10.1155/2013/732742.
Horwitz, E. M., Gordon, P. L., Koo, W. K., et al. (2002). Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proceedings of the National Academy of Sciences of the United States of America, 99(13), 8932–8937.
Alm, J. J., Koivu, H. M., Heino, T. J., Hentunen, T. A., Laitinen, S., & Aro, H. T. (2010). Circulating plastic adherent mesenchymal stem cells in aged hip fracture patients. Journal of Orthopaedic Research, 28(12), 1634–1642.
Granero-Molto, F., Weis, J. A., Miga, M. I., et al. (2009). Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells, 27(8), 1887–1898.
Shirley, D., Marsh, D., Jordan, G., McQuaid, S., & Li, G. (2005). Systemic recruitment of osteoblastic cells in fracture healing. Journal of Orthopaedic Research, 23(5), 1013–1021.
Kolios, L., Hoerster, A. K., Sehmisch, S., et al. (2010). Do estrogen and alendronate improve metaphyseal fracture healing when applied as osteoporosis prophylaxis? Calcified Tissue International, 86(1), 23–32.
Westerlind, K. C., Wronski, T. J., Ritman, E. L., et al. (1997). Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proceedings of the National Academy of Sciences of the United States of America, 94(8), 4199–4204.
Squire, M., Brazin, A., Keng, Y., & Judex, S. (2008). Baseline bone morphometry and cellular activity modulate the degree of bone loss in the appendicular skeleton during disuse. Bone, 42(2), 341–349.
Carod Artal, F. J. (1995). What should first be considered in the treatment of epileptic patients: control of seizures or quality of life? Revista de Neurologia, 23(124), 1325.
McCann, R. M., Colleary, G., Geddis, C., et al. (2008). Effect of osteoporosis on bone mineral density and fracture repair in a rat femoral fracture model. Journal of Orthopaedic Research, 26(3), 384–393.
Hao, Y. J., Zhang, G., Wang, Y. S., et al. (2007). Changes of microstructure and mineralized tissue in the middle and late phase of osteoporotic fracture healing in rats. Bone, 41(4), 631–638.
Augat P., Simon U., Liedert A., Claes L., (2005). Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporosis International, Suppl 2:S36-S43
Barrios, C., Brostrom, L. A., Stark, A., & Walheim, G. (1993). Healing complications after internal fixation of trochanteric hip fractures: the prognostic value of osteoporosis. Journal of Orthopaedic Trauma, 7(5), 438–442.
Hong, L., Zhang, G., Sultana, H., Yu, Y., & Wei, Z. (2011). The effects of 17-beta estradiol on enhancing proliferation of human bone marrow mesenchymal stromal cells in vitro. Stem Cells and Development, 20(5), 925–931.
Turner, R. T., Riggs, B. L., & Spelsberg, T. C. (1994). Skeletal effects of estrogen. Endocrine Reviews, 15(3), 275–300.
Zhang, L., & Chan, C. (2010). Isolation and enrichment of rat mesenchymal stem cells (MSCs) and separation of single-colony derived MSCs. Journal of Visualized Experiments. doi:10.3791/1852.
Gruber, H. E., Somayaji, S., Riley, F., et al. (2012). Human adipose-derived mesenchymal stem cells: serial passaging, doubling time and cell senescence. Biotechnic & Histochemistry, 87(4), 303–311.
Bhargavan, B., Gautam, A. K., Singh, D., et al. (2009). Methoxylated isoflavones, cajanin and isoformononetin, have non-estrogenic bone forming effect via differential mitogen activated protein kinase (MAPK) signaling. Journal of Cellular Biochemistry, 108(2), 388–399. doi:10.1002/jcb.22264.
Swarnkar, G., Sharan, K., Siddiqui, J. A., et al. (2011). A novel flavonoid isolated from the steam-bark of Ulmus wallichiana planchon stimulates osteoblast function and inhibits osteoclast and adipocyte differentiation. European Journal of Pharmacology, 658(2–3), 65–73.
Khan, K., Sharan, K., Swarnkar, G., et al. (2013). Positive skeletal effects of cladrin, a naturally occurring dimethoxydaidzein, in osteopenic rats that were maintained after treatment discontinuation. Osteoporosis International, 24, 1455–1470. doi:10.1007/s00198-012-2121-8.
Abbas, S., Khan, K., Khan, M. P., et al. (2013). Developmental exposure to As, Cd, and Pb mixture diminishes skeletal growth and causes osteopenia at maturity via osteoblast and chondrocyte malfunctioning in female rats. Toxicological Sciences, 134(1), 207–220.
Ngueguim, F. T., Khan, M. P., Donfack, J. H., et al. (2012). Evaluation of Cameroonian plants towards experimental bone regeneration. Journal of Ethnopharmacology, 141(1), 331–337.
de Vries, E. F., Roca, M., Jamar, F., Israel, O., & Signore, A. (2010). Guidelines for the labelling of leucocytes with (99 m)Tc-HMPAO. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. European Journal of Nuclear Medicine and Molecular Imaging, 37(4), 842–848.
Sharan, K., Mishra, J. S., Swarnkar, G., et al. (2011). A novel quercetin analogue from a medicinal plant promotes peak bone mass achievement and bone healing after injury and exerts an anabolic effect on osteoporotic bone: the role of aryl hydrocarbon receptor as a mediator of osteogenic action. Journal of Bone and Mineral Research, 26(9), 2096–2111.
Hildebrand, T., & Ruegsegger, P. (1997). Quantification of bone microarchitecture with the structure model index. Computer Methods in Biomechanics and Biomedical Engineering, 1(1), 15–23.
Sagar, N., Soni, V. P., & Bellare, J. R. (2012). Influence of carboxymethyl chitin on stability and biocompatibility of 3D nanohydroxyapatite/gelatin/carboxymethyl chitin composite for bone tissue engineering. Journal of Biomedical Materials Research, 100(3), 624–636.
Sagar, N., Pandey, A. K., Gurbani, D., et al. (2013). In-vivo efficacy of compliant 3D nano-composite in critical-size bone defect repair: a six month preclinical study in rabbit. PLoS ONE, 8, e77578. doi:10.1371/journal.pone.0077578.
Rai, R. K., Barbhuyan, T., Singh, C., et al. (2013). Total water, phosphorus relaxation and interatomic organic to inorganic interface are new determinants of trabecular bone integrity. PLoS ONE, 8(12), e83478. doi:10.1371/journal.pone.0083478.
Stenderup, K., Justesen, J., Clausen, C., & Kassem, M. (2003). Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone, 33(6), 919–926.
Wilson, A., Shehadeh, L. A., Yu, H., & Webster, K. A. (2010). Age-related molecular genetic changes of murine bone marrow mesenchymal stem cells. BMC Genomics, 11, 229. doi:10.1186/1471-2164-11-229.
Rodriguez, J. P., Montecinos, L., Rios, S., et al. (2000). Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. Journal of Cellular Biochemistry, 79(4), 557–565.
Gao, Y., Jiao, Y., Nie, W., et al. (2014). In vitro proliferation and differentiation potential of bone marrow-derived mesenchymal stem cells from ovariectomized rats. Tissue and Cell, S0040–8166(14), 00075–5.
Hervouet, E., Cartron, P. F., Jouvenot, M., & Delage-Mourroux, R. (2013). Epigenetic regulation of estrogen signaling in breast cancer. Epigenetics, 8(3), 237–245. doi:10.4161/epi.23790.
Ayaloglu-Butun, F., Terzioglu-Kara, E., Tokcaer-Keskin, Z., & Akcali, K. C. (2012). The effect of estrogen on bone marrow-derived rat mesenchymal stem cell maintenance: inhibiting apoptosis through the expression of Bcl-xL and Bcl-2. Stem Cell Reviews and Reports, 8(2), 393–401. doi:10.1007/s12015-011-9292-0.
Diwan, A. D., Leong, A., Appleyard, R., Bhargav, D., Fang, Z. M., & Wei, A. (2013). Bone morphogenetic protein-7 accelerates fracture healing in osteoporotic rats. Indian Journal of Orthopaedics, 47(6), 540–546.
Nebel, D., Jönsson, D., Norderyd, O., Bratthall, G., & Nilsson, B. O. (2010). Differential regulation of chemokine expression by estrogen in human periodontal ligament cells. Journal of Periodontal Research, 45(6), 796–802. doi:10.1111/j.1600-0765.2010.01308.x.
Boudot, A., Kerdivel, G., Habauzit, D., et al. (2011). Differential estrogen-regulation of CXCL12 chemokine receptors, CXCR4 and CXCR7, contributes to the growth effect of estrogens in breast cancer cells. PLoS ONE, 6(6), e20898. doi:10.1371/journal.pone.0020898.
Ji, J. F., He, B. P., Dheen, S. T., & Tay, S. S. (2004). Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells, 22(3), 415–427.
Neer, R. M., Arnaud, C. D., Zanchetta, J. R., et al. (2001). Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. The New England Journal of Medicine, 344(19), 1434–1441.
Valentin-Opran, A., Wozney, J., Csimma, C., Lilly, L., & Riedel, G. E. (2002). Clinical evaluation of recombinant human bone morphogenetic protein-2. Clinical Orthopaedics and Related Research, 2(395), 110–120.
Acknowledgments
The authors are thankful for the technical assistance provided by Kavita Singh at the Confocal Microscopy Facility, SAIF division, CSIR-CDRI.
Conflict of Interest
Although in no way related to this study, NC has received research support from GlaxoSmithKline Consumer Health Care, Gurgaon, India and served as an advisory board member of Alkem Laboratories Ltd, India. All other authors have no conflict of interest.
Funding
Authors acknowledge funding from Council of Scientific and Industrial Research (CSIR) to [N.C.] (BSC0201, ASTHI); Research fellowship grants from the Department of Biotechnology [DT], Indian Council of Medical Research [MPK] and CSIR (S.P.C).
Supporting grants
Council of Scientific and Industrial Research, Indian Council of Medical Research and Department of Biotechnology, Government of India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tewari, D., Khan, M.P., Sagar, N. et al. Ovariectomized Rats with Established Osteopenia have Diminished Mesenchymal Stem Cells in the Bone Marrow and Impaired Homing, Osteoinduction and Bone Regeneration at the Fracture Site. Stem Cell Rev and Rep 11, 309–321 (2015). https://doi.org/10.1007/s12015-014-9573-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9573-5