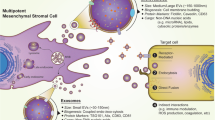
Abstract
Background
The therapeutic potential of mesenchymal stromal cells (MSCs) may be largely mediated by paracrine factors contained in microvesicles (MV) released from intracellular endosomes. A systematic review of controlled interventional animal studies was performed to identify models of organ injury where clinical translation of MSC-derived microvesicle therapy appears most promising as regenerative therapy.
Methods
A total of 190 published articles were identified in our systematic search of electronic databases (MEDLINE, EMBASE, PUBMED). After screening for eligibility, a total of 17 controlled studies testing MSC-derived MVs as therapeutic interventions in animal models of disease underwent comprehensive review, quality assessment, and data extraction.
Results
Thirteen studies addressed the regenerative potential following organ injury. Six studies were included on acute kidney injury, 4 on myocardial infarction and reperfusion injury, 1 on hind limb ischemia, 1 on liver injury, and 1 on hypoxic lung injury. Four studies addressed immunological effects of MSC-derived MVs on inhibiting tumor growth. Twelve studies (71 %) provided explicit information regarding the number of animals allocated to treatment or control groups. Five studies (29 %) randomly assigned animals to treatment or control groups and only 1 study (6 %) reported on blinding. Therapeutic intervention involved isolation of exosomes (40–100 nm) in eight studies, while nine studies tested unfractionated microvesicles (<1,000 nm). In studies of tissue regeneration, all 13 reported that treatment with MSC-derived MVs improved at least one major/clinical parameter associated with organ dysfunction. Three of 4 studies evaluating the inhibition of tumor growth reported benefit.
Conclusions
In preclinical studies, the use of MSC-derived MVs is strongly associated with improved organ function following injury and may be useful for inhibiting tumor growth. Improved preclinical study quality in terms of treatment allocation reporting, randomization and blinding will accelerate needed progress towards clinical trials that should assess feasibility and safety of this therapeutic approach in humans.


Similar content being viewed by others
References
Lalu, M. M., McIntyre, L., Pugliese, C., et al. (2012). Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PloS One, 7, e47559.
Hofmann, N. A., Ortner, A., Jacamo, R. O., et al. (2012). Oxygen sensing mesenchymal progenitors promote neo-vasculogenesis in a humanized mouse model in vivo. PloS One, 7, e44468.
Bell, G. I., Meschino, M. T., Hughes-Large, J. M., et al. (2012). Combinatorial human progenitor cell transplantation optimizes islet regeneration through secretion of paracrine factors. Stem Cells and Development, 21, 1863–1876.
Zhao, S., Wehner, R., Bornhauser, M., et al. (2010). Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells and Development, 19, 607–614.
Nauta, A. J., & Fibbe, W. E. (2007). Immunomodulatory properties of mesenchymal stromal cells. Blood, 110, 3499–3506.
Beitnes, O., Oie, E., Shahdadfar, A., et al. (2012). Intramyocardial injections of human mesenchymal stem cells following acute myocardial infarction modulate scar formation and improve left ventricular function. Cell Transplantation, 21, 1697–1709.
Zhu, X. Y., Lerman, A., & Lerman, L. O. (2013). Concise review: mesenchymal stem cell treatment for ischemic kidney disease. Stem Cells, 31, 1731–1736.
Waszak, P., Alphonse, R., Vadivel, A., et al. (2012). Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells and Development, 21, 2789–2797.
Ionescu, L., Byrne, R. N., van Haaften, T., et al. (2012). Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. American Journal of Physiology Lung Cellular and Molecular Physiology, 303, L967–L977.
Fouraschen, S. M., Pan, Q., de Ruiter, P. E., et al. (2012). Secreted factors of human-derived mesenchymal stem cells promote liver regeneration early after partial hepatectomy. Stem Cells and Development, 21, 2410–2419.
Lai, R. C., Chen, T. S., & Lim, S. K. (2011). Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regenerative Medicine, 6, 481–492.
Camussi, G., Deregibus, M. C., & Tetta, C. (2010). Paracrine/endocrine mechanism of stem cells on kidney repair: role of microvesicle-mediated transfer of genetic information. Current Opinion Nephrol Hypertension, 19, 7–12.
Im, H., Shao, H., Park, Y. I., et al. (2014). Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nature Biotechnology. doi:10.1038/nbt.2886. Epub ahead of print April 20, 2014.
Robbins, P. D., & Morelli, A. E. (2014). Regulation of immune responses by extracellular vesicles. Nature Reviews Immunology, 14, 195–208.
Lee, J. K., Park, S. R., Jung, B. K., et al. (2013). Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PloS One, 8, e84256.
Roccaro, A. M., Sacco, A., Maiso, P., et al. (2013). BM Mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. Journal of Clinical Investigation, 123, 1542–1555.
Looze, C., Yui, D., Leung, L., et al. (2009). Proteomic profiling of human plasma exosomes identifies PPARgamma as an exosome-associated protein. Biochemical and Biophysical Research Communications, 378, 433–438.
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., & PRISMA Group. (2009). Preferred reporting items for sytematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine, 6, e1000097.
Henderson, V. C., Kimmelman, J., Fergusson, D., Grimshaw, J. M., & Hackam, D. G. (2013). Threats to validity in the design and conduct of preclinical efficacy studies: a systematic review of guidelines for in vivo animal experiments. PLoS Medicine, 10, e1001489.
Bruno, S., Grange, C., Deregibus, M. C., Calogero, R. A., Saviozzi, S., Collino, F., et al. (2009). Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. Journal of the American Society of Nephrology, 20, 1053–1067.
Bruno, S., Grange, C., Collino, F., Deregibus, M. C., Cantaluppi, V., Biancone, L., et al. (2012). Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PloS One, 7, e33115.
Gatti, S., Bruno, S., Deregibus, M. C., Sordi, A., Cantaluppi, V., Tetta, C., et al. (2011). Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrology Dialysis Transplantation, 26, 1474–1483.
He, J., Wang, Y., Sun, S., Yu, M., Wang, C., Pei, X., et al. (2012). Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology, 17, 493–500.
Reis, L. A., Borges, F. T., Simoes, M. J., Borges, A. A., Sinigaglia-Coimbra, R., & Schor, N. (2012). Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PloS One, 7, e44092.
Zhou, Y., Xu, H., Xu, W., Wang, B., Wu, H., Tao, Y., et al. (2013). Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Research Therapy, 4, 34.
Arslan, F., Lai, R. C., Smeets, M. B., Akeroyd, L., Choo, A., Aguor, E. N. E., et al. (2013). Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Research, 10, 301–312.
Lai, R. C., Arslan, F., Lee, M. M., Sze, N. S. K., Choo, A., Chen, T. S., et al. (2010). Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Research, 4, 214–222.
Lai, R. C., Arslan, F., Tan, S. S., Tan, B., Choo, A., Lee, M. M., et al. (2010). Derivation and characterization of human fetal MSCs: an alternative cell source for large-scale production of cardioprotective microparticles. Journal of Molecular and Cellular Cardiology, 48, 1215–1224.
Chen, T. S., Lai, R. C., Lee, M. M., et al. (2010). Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Research, 38, 215–224.
Zhang, H. C., Liu, X. B., Huang, S., Bi, X. Y., Wang, H. X., Xie, L. X., et al. (2012). Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells and Development, 21, 3289–3297.
Li, T., Yan, Y., Wang, B., Qian, H., Zhang, X., Shen, L., et al. (2013). Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells and Development, 22, 845–854.
Lee, C., Mitsialis, S. A., Aslam, M., Vitali, S. H., Vergadi, E., Konstantinou, G., et al. (2012). Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation, 126, 2601–2611.
Bruno, S., Collino, F., Deregibus, M. C., Grange, C., Tetta, C., & Camussi, G. (2013). Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells and Development, 22, 758–771.
Zhu, W., Huang, L., Li, Y., Zhang, X., Gu, J., Yan, Y., et al. (2012). Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Letters, 315, 28–37.
Wu, S., Ju, G. Q., Du, T., Zhu, Y. J., & Liu, G. H. (2013). Microvesicles derived from human umbilical cord Wharton’s jelly mesenchymal stem cells attenuate bladder tumor cell growth in vitro and in vivo. PloS One, 8, e61366.
Rosu-Myles M, Gillham-Eisen LA, Agbanyo FR, Ganz PR (2011) Regulatory questions in the development of blood stem cell products for regenerative therapy. In: Regenerative Therapy Using Blood-Derived Stem Cells ed. DS Allan and D Strunk, Springer Science, pp 167–190
Bernardo, M. E., & Fibbe, W. E. (2012). Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Annals of the New York Academy of Sciences, 1266, 107–117.
von Bahr, L., Batsis, I., Moll, G., et al. (2012). Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells, 30, 1575–1578.
Clark, E. A., Kalomoiris, S., Nolta, J. A., & Fierro, F. A. (2013). MicroRNA function in multipotent mesenchymal stromal cells. Stem Cells, 32, 1074–1082.
Lavoie, J. R., & Rosu-Myles, M. (2013). Uncovering the secretes of mesenchymal stem cells. Biochimie, 95, 2212–2221.
Acknowledgments
We wish to acknowledge the expertise and assistance of Risa Shorr from the library at The Ottawa Hospital for help with design and execution of the systematic search. Funding support for CA was provided by The Ottawa Hospital Foundation Research Fund for Hematology and Blood and Marrow Transplantation. Salary support from a New Investigator Award (DSA) and Mentorship Award in Clinical Trials (JT) was generously provided by Canadian Institutes of Health Research. An endowed Chair in Clinical Epidemiology (DF) from the University of Ottawa (U of O) and Ottawa Hospital Research Institute is gratefully acknowledged. DSA and JT are supported in part by the Department of Medicine at U of O. Part of this work was undertaken as part of the Science and Technology program of the Canadian Government, AECL Project 1.4.4-8 Improving Occupational Dosimetry.
Disclosures
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akyurekli, C., Le, Y., Richardson, R.B. et al. A Systematic Review of Preclinical Studies on the Therapeutic Potential of Mesenchymal Stromal Cell-Derived Microvesicles. Stem Cell Rev and Rep 11, 150–160 (2015). https://doi.org/10.1007/s12015-014-9545-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9545-9