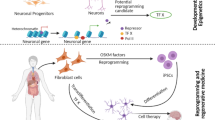
Abstract
The crucial facts underlying the low efficiency of cellular reprogramming are poorly understood. Cellular reprogramming occurs in nuclear transfer, induced pluripotent stem cell (iPSC) formation, cell fusion, and lineage-switching experiments. Despite these advances, there are three fundamental problems to be addressed: (1) the majority of cells cannot be reprogrammed, (2) the efficiency of reprogramming cells is usually low, and (3) the reprogrammed cells developed from a patient’s own cells activate immune responses. These shortcomings present major obstacles for using reprogramming approaches in customised cell therapy. In this Perspective, the author synthesises past and present observations in the field of cellular reprogramming to propose a theoretical picture of the cellular memory disc. The current hypothesis is that all cells undergo an endogenous and exogenous holographic memorisation such that parts of the cellular memory dramatically decrease the efficiency of reprogramming cells, act like a barrier against reprogramming in the majority of cells, and activate immune responses. Accordingly, the focus of this review is mainly to describe the cellular memory disc (CMD). Based on the present theory, cellular memory includes three parts: a reprogramming-resistance memory (RRM), a switch-promoting memory (SPM) and a culture-induced memory (CIM), which arises genetically, epigenetically and non-genetically, respectively, and affect cellular behaviours.








Similar content being viewed by others
References
Gaspar-Maia, A., Alajem, A., Meshorer, E., & Ramalho-Santos, M. (2011). Open chromatin in pluripotency and reprogramming. Nature Reviews Molecular Cell Biology, 12(1), 36–47.
Nagy, A., Gócza, E., Diaz, E. M., Prideaux, V. R., Iványi, E., Markkula, M., et al. (1990). Embryonic stem cells alone are able to support fetal development in the mouse. Development, 110(3), 815–821.
Kriegstein, A., & Alvarez-Buylla, A. (2009). The glial nature of embryonic and adult neural stem cells. Annual Review of Neuroscience, 32, 149–184.
Cibelli, J. B., Grant, K. A., Chapman, K. B., Cunniff, K., Worst, T., Green, H. L., et al. (2002). Parthenogenetic stem cells in nonhuman primates. Science, 295(5556), 819.
Gurdon, J. B., & Melton, D. A. (2008). Nuclear reprogramming in cells. Science, 322(5909), 1811–1815.
Byrne, J. A., Pedersen, D. A., Clepper, L. L., Nelson, M., Sanger, W. G., Gokhale, S., et al. (2007). Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature, 450(7169), 497–502.
Solter, D. (2006). From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nature Reviews Genetics, 7(4), 319–327.
Zwaka, T. P., & Thomson, J. A. (2005). A germ cell origin of embryonic stem cells? Development, 132(2), 227–233.
Cowan, C. A., Atienza, J., Melton, D. A., & Eggan, K. (2005). Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science, 309(5739), 1369–1373.
Jaenisch, R., & Young, R. (2008). Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell, 132(4), 567–582.
Tosh, D., & Slack, J. M. (2002). How cells change their phenotype. Nature Reviews Molecular Cell Biology, 3(3), 187–194.
Kagias, K., Ahier, A., Fischer, N., & Jarriault, S. (2012). Members of the NODE (Nanog and Oct4-associated deacetylase) complex and SOX-2 promote the initiation of a natural cellular reprogramming event in vivo. Proceedings of the National Academy of Sciences of the United States of America, 109(17), 6596–6601.
McKay, R. (2000). Stem cells–hype and hope. Nature, 406(6794), 361–364.
Gurdon, J. B., Byrne, J. A., & Simonsson, S. (2003). Nuclear reprogramming and stem cell creation. Proceedings of the National Academy of Sciences of the United States of America, 100(Suppl 1), 11819–11822.
Park, I. H., Arora, N., Huo, H., Maherali, N., Ahfeldt, T., Shimamura, A., et al. (2008). Disease-specific induced pluripotent stem cells. Cell, 134(5), 877–886.
Dimos, J. T., Rodolfa, K. T., Niakan, K. K., Weisenthal, L. M., Mitsumoto, H., Chung, W., et al. (2008). Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science, 321(5893), 1218–1221.
Israel, M. A., Yuan, S. H., Bardy, C., Reyna, S. M., Mu, Y., Herrera, C., et al. (2012). Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature, 482(7384), 216–220.
Lujan, E., Chanda, S., Ahlenius, H., Südhof, T. C., & Wernig, M. (2012). Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proceedings of the National Academy of Sciences of the United States of America, 109(7), 2527–2532.
Ng, R. K., & Gurdon, J. B. (2008). Epigenetic memory of an active gene state depends on histone H3.3 incorporation into chromatin in the absence of transcription. Nature Cell Biology, 10(1), 102–109.
Kim, K., Doi, A., Wen, B., Ng, K., Zhao, R., Cahan, P., et al. (2010). Epigenetic memory in induced pluripotent stem cells. Nature, 467(7313), 285–290.
Polo, J. M., Liu, S., Figueroa, M. E., Kulalert, W., Eminli, S., Tan, K. Y., et al. (2010). Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nature Biotechnology, 28(8), 848–855.
Zhao, T., Zhang, Z. N., Rong, Z., & Xu, Y. (2011). Immunogenicity of induced pluripotent stem cells. Nature, 474(7350), 212–215.
Levenson, J. M., & Sweatt, J. D. (2005). Epigenetic mechanisms in memory formation. Nature Reviews Neuroscience, 6(2), 108–118.
Hochedlinger, K., & Jaenisch, R. (2007). On the cloning of animals from terminally differentiated cells. Nature Genetics, 39(2), 136–137.
Meissner, A., & Jaenisch, R. (2006). Mammalian nuclear transfer. Developmental Dynamics, 235(9), 2460–2469.
Meissner, A., Wernig, M., & Jaenisch, R. (2007). Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nature Biotechnology, 25(10), 1177–1181.
Plath, K., & Lowry, W. E. (2011). Progress in understanding reprogramming to the induced pluripotent state. Nature Reviews Genetics, 12(4), 253–265.
Zhou, Q., Brown, J., Kanarek, A., Rajagopal, J., & Melton, D. A. (2008). In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature, 455(7213), 627–632.
Gurdon, J. B. (2006). From nuclear transfer to nuclear reprogramming: the reversal of cell differentiation. Annual Review of Cell and Developmental Biology, 22, 1–22.
Lluis, F., Pedone, E., Pepe, S., & Cosma, M. P. (2010). The Wnt/β-catenin signaling pathway tips the balance between apoptosis and reprograming of cell fusion hybrids. Stem Cells, 28(11), 1940–1949.
Breiling, A., Turner, B. M., Bianchi, M. E., & Orlando, V. (2001). General transcription factors bind promoters repressed by Polycomb group proteins. Nature, 412(6847), 651–655.
Dodd, I. B., Micheelsen, M. A., Sneppen, K., & Thon, G. (2007). Theoretical analysis of epigenetic cell memory by nucleosome modification. Cell, 129(4), 813–822.
Segal, E., Fondufe-Mittendorf, Y., Chen, L., Thåström, A., Field, Y., Moore, I. K., et al. (2006). A genomic code for nucleosome positioning. Nature, 442(7104), 772–778.
van Oosten, A. L., Costa, Y., Smith, A., & Silva, J. C. (2012). JAK/STAT3 signalling is sufficient and dominant over antagonistic cues for the establishment of naive pluripotency. Nature Communications, 3, 817.
Bird, A. (2007). Perceptions of epigenetics. Nature, 447(7143), 396–398.
Kim, J., Efe, J. A., Zhu, S., Talantova, M., Yuan, X., Wang, S., et al. (2011). Direct reprogramming of mouse fibroblasts to neural progenitors. Proceedings of the National Academy of Sciences of the United States of America, 108(19), 7838–7843.
Stadtfeld, M., Apostolou, E., Ferrari, F., Choi, J., Walsh, R. M., Chen, T., et al. (2012). Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nature Genetics, 44(4), 398–405.
Esteban, M. A., & Pei, D. (2012). Vitamin C improves the quality of somatic cell reprogramming. Nature Genetics, 44(4), 366–367.
Anjamrooz, S. H. (2011). Trinity is a numerical model of the holographic universe. Int J Phys Sci., 6(2), 175–181.
Anjamrooz, S. H., McConnell, D. J., & Azari, H. (2011). The cellular universe: a new cosmological model based on the holographic principle. Int J Phys Sci., 6(9), 2175–2183.
Sheppard, T. L., Ordoukhanian, P., & Joyce, G. F. (2000). A DNA enzyme with N-glycosylase activity. Proceedings of the National Academy of Sciences of the United States of America, 97(14), 7802–7807.
Hyden, H., & Egyhazi, E. (1962). Nuclear RNA changes of nerve cells during a learning experiment in rats. Proceedings of the National Academy of Sciences of the United States of America, 48, 1366–1373.
Babich, F. R., Jacobson, A. L., Bubash, S., & Jacobson, A. (1965). Transfer of a response to naive rats by injection of ribonucleic acid extracted from trained rats. Science, 149(3684), 656–657.
Jacobson, A. L., Babich, F. R., Bubash, S., & Jacobson, A. (1965). Differential-approach tendencies produced by injection of RNA from trained rats. Science, 150(3696), 636–637.
McConnell, J. V., Jacobson, A. L., & Kimble, D. P. (1959). The effects of regeneration upon retention of a conditioned response in the planarian. Journal of Comparative and Physiological Psychology, 52(1), 1–5.
Corning, W. C., & John, E. R. (1961). Effect of ribonuclease on retention of conditioned response in regenerated planarians. Science, 134(3487), 1363–1365.
Niu, M. C. (1958). Thymus ribonucleic acid and embryonic differentiation. Proceedings of the National Academy of Sciences of the United States of America, 44(12), 1264–1274.
Fishman, M., Hammerstrom, R. A., & Bond, V. P. (1963). In vitro transfer of macrophage RNA to lymph node cells. Nature, 198, 549–551.
Brewer, R. G., & Hahn, E. L. (1984). Atomic memory. Scientific American, 251, 50–57.
Yoshikawa, Y., Nakayama, K., Torii, Y., & Kuga, T. (2007). Holographic storage of multiple coherence gratings in a Bose-Einstein condensate. Physical Review Letters, 99(22), 220407.
Shipway, A. N., Katz, E., & Willner, I. (2001). Molecular memory and processing devices in solution and on surfaces. Struct. Bonding., 99, 237–281.
Berchtold, N. C., Chinn, G., Chou, M., Kesslak, J. P., & Cotman, C. W. (2005). Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience, 133(3), 853–861.
Shinde, U. P., Liu, J. J., & Inouye, M. (1997). Protein memory through altered folding mediated by intramolecular chaperones. Nature, 389(6650), 520–522.
Bentolila, S. (2005). “Live memory” of the cell, the other hereditary memory of living systems. Biosystems, 80(3), 251–261.
Fesenko, E. E., Geletyuk, V. I., Kazachenko, V. N., & Chemeris, N. K. (1995). Preliminary microwave irradiation of water solutions changes their channel-modifying activity. FEBS Letters, 366(1), 49–52.
Maddox, J. (1988). When to believe the unbelievable. Nature, 333, 816–818.
Samal, S., & Geckeler, K. E. (2001). Unexpected solute aggregation in water on dilution. Chemical Communications (Cambridge, England), 7(21), 2224–2225.
Del Giudice, E., Preparata, G., & Vitiello, G. (1988). Water as a free electric dipole laser. Physical Review Letters, 61(9), 1085–1088.
Ahn, J., Weinacht, T. C., & Bucksbaum, P. H. (2000). Information storage and retrieval through quantum phase. Science, 287(5452), 463–465.
Davenas, E., Beauvais, F., Amara, J., Oberbaum, M., Robinzon, B., Miadonna, A., et al. (1988). Human basophil degranulation triggered by very dilute antiserum against IgE. Nature, 333(6176), 816–818.
Farhadi, A., Forsyth, C., Banan, A., Shaikh, M., Engen, P., Fields, J. Z., et al. (2007). Evidence for non-chemical, non-electrical intercellular signaling in intestinal epithelial cells. Bioelectrochemistry, 71(2), 142–148.
Gabor, D. (1968). Holographic model of temporal recall. Nature, 217(5128), 584.
Greguss, P. (1968). Bioholography—a new model of information processing. Nature, 219(5153), 482.
Klonguet-Higgins, H. C. (1968). Holographic model of temporal recall. Nature, 217(5123), 104.
Greer, E. L., & Shi, Y. (2012). Histone methylation: a dynamic mark in health, disease and inheritance. Nature Reviews Genetics, 13(5), 343–357.
Greer, E. L., Maures, T. J., Ucar, D., Hauswirth, A. G., Mancini, E., Lim, J. P., et al. (2011). Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature, 479(7373), 365–371.
Zuber, J., Shi, J., Wang, E., Rappaport, A. R., Herrmann, H., Sison, E. A., et al. (2011). RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature, 478(7370), 524–528.
Barrero, M. J., & Izpisua Belmonte, J. C. (2011). iPS cells forgive but do not forget. Nature Cell Biology, 13(5), 523–525.
Okano, M., Xie, S., & Li, E. (1998). Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nature Genetics, 19(3), 219–220.
Bhutani, N., Brady, J. J., Damian, M., Sacco, A., Corbel, S. Y., & Blau, H. M. (2010). Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature, 463(7284), 1042–1047.
Simonsson, S., & Gurdon, J. (2004). DNA demethylation is necessary for the epigenetic reprogramming of somatic cell nuclei. Nature Cell Biology, 6(10), 984–990.
Mikkelsen, T. S., Hanna, J., Zhang, X., Ku, M., Wernig, M., Schorderet, P., et al. (2008). Dissecting direct reprogramming through integrative genomic analysis. Nature, 454(7200), 49–55.
Popp, C., Dean, W., Feng, S., Cokus, S. J., Andrews, S., Pellegrini, M., et al. (2010). Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature, 463(7284), 1101–1105.
Lessard, J. A., & Crabtree, G. R. (2010). Chromatin regulatory mechanisms in pluripotency. Annual Review of Cell and Developmental Biology, 26, 503–532.
Luger, K., Mäder, A. W., Richmond, R. K., Sargent, D. F., & Richmond, T. J. (1997). Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature, 389(6648), 251–260.
van Leeuwen, F., Gafken, P. R., & Gottschling, D. E. (2002). Dot1p modulates silencing in yeast by methylation of the nucleosome core. Cell, 109(6), 745–756.
Dorigo, B., Schalch, T., Kulangara, A., Duda, S., Schroeder, R. R., & Richmond, T. J. (2004). Nucleosome arrays reveal the two-start organization of the chromatin fiber. Science, 306(5701), 1571–1573.
Atkins, C. M., Selcher, J. C., Petraitis, J. J., Trzaskos, J. M., & Sweatt, J. D. (1998). The MAPK cascade is required for mammalian associative learning. Nature Neuroscience, 1(7), 602–609.
Rampon, C., Tang, Y. P., Goodhouse, J., Shimizu, E., Kyin, M., & Tsien, J. Z. (2000). Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nature Neuroscience, 3(3), 238–244.
Malenka, R. C., & Bear, M. F. (2004). LTP and LTD: an embarrassment of riches. Neuron, 44(1), 5–21.
Klann, E., Antion, M. D., Banko, J. L., & Hou, L. (2004). Synaptic plasticity and translation initiation. Learning and Memory, 11(4), 365–372.
Pittenger, C., & Kandel, E. R. (2003). In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358(1432), 757–763.
Korzus, E., Rosenfeld, M. G., & Mayford, M. (2004). CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron, 42(6), 961–972.
Alarcón, J. M., Malleret, G., Touzani, K., Vronskaya, S., Ishii, S., Kandel, E. R., et al. (2004). Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron, 42(6), 947–959.
Levenson, J. M., O’Riordan, K. J., Brown, K. D., Trinh, M. A., Molfese, D. L., & Sweatt, J. D. (2004). Regulation of histone acetylation during memory formation in the hippocampus. Journal of Biological Chemistry, 279(39), 40545–40559.
Yeh, S. H., Lin, C. H., & Gean, P. W. (2004). Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Molecular Pharmacology, 65(5), 1286–1292.
Weaver, I. C., Cervoni, N., Champagne, F. A., D’Alessio, A. C., Sharma, S., Seckl, J. R., et al. (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7(8), 847–854.
Kaminsky, Z. A., Tang, T., Wang, S. C., Ptak, C., Oh, G. H., Wong, A. H., et al. (2009). DNA methylation profiles in monozygotic and dizygotic twins. Nature Genetics, 41(2), 240–245.
Fraga, M. F., Ballestar, E., Paz, M. F., Ropero, S., Setien, F., Ballestar, M. L., et al. (2005). Epigenetic differences arise during the lifetime of monozygotic twins. Proceedings of the National Academy of Sciences of the United States of America, 102(30), 10604–10609.
Henderson, I. R., Shindo, C., & Dean, C. (2003). The need for winter in the switch to flowering. Annual Review of Genetics, 37, 371–392.
Reppert, S. M., & Weaver, D. R. (2002). Coordination of circadian timing in mammals. Nature, 418(6901), 935–941.
Naruse, Y., Oh-hashi, K., Iijima, N., Naruse, M., Yoshioka, H., & Tanaka, M. (2004). Circadian and light-induced transcription of clock gene Per1 depends on histone acetylation and deacetylation. Molecular and Cellular Biology, 24(14), 6278–6287.
Crosio, C., Cermakian, N., Allis, C. D., & Sassone-Corsi, P. (2000). Light induces chromatin modification in cells of the mammalian circadian clock. Nature Neuroscience, 3(12), 1241–1247.
Hochedlinger, K., & Jaenisch, R. (2003). Nuclear transplantation, embryonic stem cells, and the potential for cell therapy. The New England Journal of Medicine, 349(3), 275–286.
Yang, X., Smith, S. L., Tian, X. C., Lewin, H. A., Renard, J. P., & Wakayama, T. (2007). Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning. Nature Genetics, 39(3), 295–302.
Ohi, Y., Qin, H., Hong, C., Blouin, L., Polo, J. M., Guo, T., et al. (2011). Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nature Cell Biology, 13(5), 541–549.
Lister, R., Pelizzola, M., Kida, Y. S., Hawkins, R. D., Nery, J. R., Hon, G., et al. (2011). Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature, 471(7336), 68–73.
McGrath, J., & Solter, D. (1984). Inability of mouse blastomere nuclei transferred to enucleated zygotes to support development in vitro. Science, 226(4680), 1317–1319.
Wilmut, I., Schnieke, A. E., McWhir, J., Kind, A. J., & Campbell, K. H. (1997). Viable offspring derived from fetal and adult mammalian cells. Nature, 385(6619), 810–813.
Jullien, J., Astrand, C., Halley-Stott, R. P., Garrett, N., & Gurdon, J. B. (2010). Characterization of somatic cell nuclear reprogramming by oocytes in which a linker histone is required for pluripotency gene reactivation. Proceedings of the National Academy of Sciences of the United States of America, 107(12), 5483–5488.
Hanna, J., Saha, K., Pando, B., van Zon, J., Lengner, C. J., Creyghton, M. P., et al. (2009). Direct cell reprogramming is a stochastic process amenable to acceleration. Nature, 462(7273), 595–601.
Brambrink, T., Foreman, R., Welstead, G. G., Lengner, C. J., Wernig, M., Suh, H., et al. (2008). Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell, 2(2), 151–159.
Pasque, V., Jullien, J., Miyamoto, K., Halley-Stott, R. P., & Gurdon, J. B. (2011). Epigenetic factors influencing resistance to nuclear reprogramming. Trends in Genetics, 27(12), 516–525.
Briggs, R., & King, T. J. (1952). Transplantation of living nuclei from blastula cells into enucleated frogs’ eggs. Proceedings of the National Academy of Sciences of the United States of America, 38(5), 455–463.
Gurdon, J. B. (1962). The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Journal of Embryology and Experimental Morphology, 10, 622–640.
Halley-Stott, R. P., Pasque, V., Astrand, C., Miyamoto, K., Simeoni, I., Jullien, J., et al. (2010). Mammalian nuclear transplantation to Germinal Vesicle stage Xenopus oocytes - a method for quantitative transcriptional reprogramming. Methods, 51(1), 56–65.
Li, J., Greco, V., Guasch, G., Fuchs, E., & Mombaerts, P. (2007). Mice cloned from skin cells. Proceedings of the National Academy of Sciences of the United States of America, 104(8), 2738–2743.
Utikal, J., Polo, J. M., Stadtfeld, M., Maherali, N., Kulalert, W., Walsh, R. M., et al. (2009). Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature, 460(7259), 1145–1148.
Egli, D., Rosains, J., Birkhoff, G., & Eggan, K. (2007). Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature, 447(7145), 679–685.
Tecirlioglu, R. T., Guo, J., & Trounson, A. O. (2006). Interspecies somatic cell nuclear transfer and preliminary data for horse-cow/mouse iSCNT. Stem Cell Reviews, 2(4), 277–287.
Weintraub, H., Tapscott, S. J., Davis, R. L., Thayer, M. J., Adam, M. A., Lassar, A. B., et al. (1989). Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proceedings of the National Academy of Sciences of the United States of America, 86(14), 5434–5438.
Wilmut, I., Beaujean, N., de Sousa, P. A., Dinnyes, A., King, T. J., Paterson, L. A., et al. (2002). Somatic cell nuclear transfer. Nature, 419(6907), 583–586.
Silva, J., Chambers, I., Pollard, S., & Smith, A. (2006). Nanog promotes transfer of pluripotency after cell fusion. Nature, 441(7096), 997–1001.
Blau, H. M., Chiu, C. P., & Webster, C. (1983). Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell, 32(4), 1171–1180.
Do, J. T., & Schöler, H. R. (2004). Nuclei of embryonic stem cells reprogram somatic cells. Stem Cells, 22(6), 941–949.
Johansson, C. B., Youssef, S., Koleckar, K., Holbrook, C., Doyonnas, R., Corbel, S. Y., et al. (2008). Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nature Cell Biology, 10(5), 575–583.
Pereira, C. F., Piccolo, F. M., Tsubouchi, T., Sauer, S., Ryan, N. K., Bruno, L., et al. (2010). ESCs require PRC2 to direct the successful reprogramming of differentiated cells toward pluripotency. Cell Stem Cell, 6(6), 547–556.
Pomerantz, J., & Blau, H. M. (2004). Nuclear reprogramming: a key to stem cell function in regenerative medicine. Nature Cell Biology, 6(9), 810–816.
Ying, Q. L., Nichols, J., Evans, E. P., & Smith, A. G. (2002). Changing potency by spontaneous fusion. Nature, 416(6880), 545–548.
Terada, N., Hamazaki, T., Oka, M., Hoki, M., Mastalerz, D. M., Nakano, Y., et al. (2002). Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature, 416(6880), 542–545.
Rudel, D., & Sommer, R. J. (2003). The evolution of developmental mechanisms. Developmental Biology, 264(1), 15–37.
Kirk, M. M., Ransick, A., McRae, S. E., & Kirk, D. L. (1993). The relationship between cell size and cell fate in Volvox carteri. The Journal of Cell Biology, 123(1), 191–208.
Lee, G., Papapetrou, E. P., Kim, H., Chambers, S. M., Tomishima, M. J., Fasano, C. A., et al. (2009). Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature, 461(7262), 402–406.
Carvajal-Vergara, X., Sevilla, A., D’Souza, S. L., Ang, Y. S., Schaniel, C., Lee, D. F., et al. (2010). Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature, 465(7299), 808–812.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676.
Kim, J. B., Sebastiano, V., Wu, G., Araúzo-Bravo, M. J., Sasse, P., Gentile, L., et al. (2009). Oct4-induced pluripotency in adult neural stem cells. Cell, 136(3), 411–419.
Anokye-Danso, F., Trivedi, C. M., Juhr, D., Gupta, M., Cui, Z., Tian, Y., et al. (2011). Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell, 8(4), 376–388.
Park, I. H., Zhao, R., West, J. A., Yabuuchi, A., Huo, H., Ince, T. A., et al. (2008). Reprogramming of human somatic cells to pluripotency with defined factors. Nature, 451(7175), 141–146.
Maekawa, M., Yamaguchi, K., Nakamura, T., Shibukawa, R., Kodanaka, I., Ichisaka, T., et al. (2011). Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature, 474(7350), 225–229.
Choi, Y. J., Lin, C. P., Ho, J. J., He, X., Okada, N., Bu, P., et al. (2011). miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nature Cell Biology, 13(11), 1353–1360.
Nakagawa, M., Koyanagi, M., Tanabe, K., Takahashi, K., Ichisaka, T., Aoi, T., et al. (2008). Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature Biotechnology, 26(1), 101–106.
Yamanaka, S. (2009). Elite and stochastic models for induced pluripotent stem cell generation. Nature, 460(7251), 49–52.
Yamanaka, S., & Blau, H. M. (2010). Nuclear reprogramming to a pluripotent state by three approaches. Nature, 465(7299), 704–712.
Warren, L., Manos, P. D., Ahfeldt, T., Loh, Y. H., Li, H., Lau, F., et al. (2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell, 7(5), 618–630.
Li, H., Collado, M., Villasante, A., Strati, K., Ortega, S., Cañamero, M., et al. (2009). The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature, 460(7259), 1136–1139.
Ho, L., & Crabtree, G. R. (2010). Chromatin remodelling during development. Nature, 463(7280), 474–484.
Chin, M. H., Mason, M. J., Xie, W., Volinia, S., Singer, M., Peterson, C., et al. (2009). Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell, 5(1), 111–123.
Okita, K., Ichisaka, T., & Yamanaka, S. (2007). Generation of germline-competent induced pluripotent stem cells. Nature, 448(7151), 313–317.
Wernig, M., Meissner, A., Foreman, R., Brambrink, T., Ku, M., Hochedlinger, K., et al. (2007). In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature, 448(7151), 318–324.
Kanatsu-Shinohara, M., Inoue, K., Lee, J., Yoshimoto, M., Ogonuki, N., Miki, H., et al. (2004). Generation of pluripotent stem cells from neonatal mouse testis. Cell, 119(7), 1001–1012.
Guan, K., Nayernia, K., Maier, L. S., Wagner, S., Dressel, R., Lee, J. H., et al. (2006). Pluripotency of spermatogonial stem cells from adult mouse testis. Nature, 440(7088), 1199–1203.
Stadtfeld, M., Maherali, N., Breault, D. T., & Hochedlinger, K. (2008). Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell, 2(3), 230–240.
Smith, A. G., Heath, J. K., Donaldson, D. D., Wong, G. G., Moreau, J., Stahl, M., et al. (1988). Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature, 336(6200), 688–690.
Ogawa, K., Nishinakamura, R., Iwamatsu, Y., Shimosato, D., & Niwa, H. (2006). Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochemical and Biophysical Research Communications, 343(1), 159–166.
Chambers, I., & Smith, A. (2004). Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene, 23(43), 7150–7160.
Ben-Shushan, E., Sharir, H., Pikarsky, E., & Bergman, Y. (1995). A dynamic balance between ARP-1/COUP-TFII, EAR-3/COUP-TFI, and retinoic acid receptor:retinoid X receptor heterodimers regulates Oct-3/4 expression in embryonal carcinoma cells. Molecular and Cellular Biology, 15(2), 1034–1048.
Fuhrmann, G., Chung, A. C., Jackson, K. J., Hummelke, G., Baniahmad, A., Sutter, J., et al. (2001). Mouse germline restriction of Oct4 expression by germ cell nuclear factor. Developmental Cell, 1(3), 377–387.
Gu, P., LeMenuet, D., Chung, A. C., Mancini, M., Wheeler, D. A., & Cooney, A. J. (2005). Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. Molecular and Cellular Biology, 25(19), 8507–8519.
Gu, P., Le Menuet, D., Chung, A. C., & Cooney, A. J. (2006). Differential recruitment of methylated CpG binding domains by the orphan receptor GCNF initiates the repression and silencing of Oct4 expression. Molecular and Cellular Biology, 26(24), 9471–9483.
Okamoto, K., Okazawa, H., Okuda, A., Sakai, M., Muramatsu, M., & Hamada, H. (1990). A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell, 60(3), 461–472.
Chin, M. H., Pellegrini, M., Plath, K., & Lowry, W. E. (2010). Molecular analyses of human induced pluripotent stem cells and embryonic stem cells. Cell Stem Cell, 7(2), 263–269.
Newman, A. M., & Cooper, J. B. (2010). Lab-specific gene expression signatures in pluripotent stem cells. Cell Stem Cell, 7(2), 258–262.
Doi, A., Park, I. H., Wen, B., Murakami, P., Aryee, M. J., Irizarry, R., et al. (2009). Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nature Genetics, 41(12), 1350–1353.
Fujiki, R., Hashiba, W., Sekine, H., Yokoyama, A., Chikanishi, T., Ito, S., et al. (2011). GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature, 480(7378), 557–560.
Silva, J., Nichols, J., Theunissen, T. W., Guo, G., van Oosten, A. L., Barrandon, O., et al. (2009). Nanog is the gateway to the pluripotent ground state. Cell, 138(4), 722–737.
Nakagawa, M., Takizawa, N., Narita, M., Ichisaka, T., & Yamanaka, S. (2010). Promotion of direct reprogramming by transformation-deficient Myc. Proceedings of the National Academy of Sciences of the United States of America, 107(32), 14152–14157.
Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318(5858), 1917–1920.
Marson, A., Foreman, R., Chevalier, B., Bilodeau, S., Kahn, M., Young, R. A., et al. (2008). Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell, 3(2), 132–135.
Feng, B., Jiang, J., Kraus, P., Ng, J. H., Heng, J. C., Chan, Y. S., et al. (2009). Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nature Cell Biology, 11(2), 197–203.
Hong, H., Takahashi, K., Ichisaka, T., Aoi, T., Kanagawa, O., Nakagawa, M., et al. (2009). Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature, 460(7259), 1132–1135.
Si-Tayeb, K., Noto, F. K., Sepac, A., Sedlic, F., Bosnjak, Z. J., Lough, J. W., et al. (2010). Generation of human induced pluripotent stem cells by simple transient transfection of plasmid DNA encoding reprogramming factors. BMC Developmental Biology, 10, 81.
Ieda, M., Fu, J. D., Delgado-Olguin, P., Vedantham, V., Hayashi, Y., Bruneau, B. G., et al. (2010). Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell, 142(3), 375–386.
Vierbuchen, T., Ostermeier, A., Pang, Z. P., Kokubu, Y., Südhof, T. C., & Wernig, M. (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature, 463(7284), 1035–1041.
Wang, L., Walker, B. L., Iannaccone, S., Bhatt, D., Kennedy, P. J., & Tse, W. T. (2009). Bistable switches control memory and plasticity in cellular differentiation. Proceedings of the National Academy of Sciences of the United States of America, 106(16), 6638–6643.
Rizzino, A. (2007). A challenge for regenerative medicine: proper genetic programming, not cellular mimicry. Developmental Dynamics, 236(12), 3199–3207.
Croft, A. P., & Przyborski, S. A. (2006). Formation of neurons by non-neural adult stem cells: potential mechanism implicates an artifact of growth in culture. Stem Cells, 24(8), 1841–1851.
Neuhuber, B., Gallo, G., Howard, L., Kostura, L., Mackay, A., & Fischer, I. (2004). Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. Journal of Neuroscience Research, 77(2), 192–204.
Joseph, N. M., & Morrison, S. J. (2005). Toward an understanding of the physiological function of Mammalian stem cells. Developmental Cell, 9(2), 173–183.
Wagers, A. J., & Weissman, I. L. (2004). Plasticity of adult stem cells. Cell, 116(5), 639–648.
Jackson, K. A., Snyder, D. S., & Goodell, M. A. (2004). Skeletal muscle fiber-specific green autofluorescence: potential for stem cell engraftment artifacts. Stem Cells, 22(2), 180–187.
Balsam, L. B., Wagers, A. J., Christensen, J. L., Kofidis, T., Weissman, I. L., & Robbins, R. C. (2004). Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature, 428(6983), 668–673.
Zhang, J., Tam, W. L., Tong, G. Q., Wu, Q., Chan, H. Y., Soh, B. S., et al. (2006). Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nature Cell Biology, 8(10), 1114–1123.
Pang, Z. P., Yang, N., Vierbuchen, T., Ostermeier, A., Fuentes, D. R., Yang, T. Q., et al. (2011). Induction of human neuronal cells by defined transcription factors. Nature, 476(7359), 220–223.
Loewer, S., Cabili, M. N., Guttman, M., Loh, Y. H., Thomas, K., Park, I. H., et al. (2010). Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nature Genetics, 42(12), 1113–1117.
Judson, R. L., Babiarz, J. E., Venere, M., & Blelloch, R. (2009). Embryonic stem cell-specific microRNAs promote induced pluripotency. Nature Biotechnology, 27(5), 459–461.
Miyoshi, N., Ishii, H., Nagano, H., Haraguchi, N., Dewi, D. L., Kano, Y., et al. (2011). Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell, 8(6), 633–638.
Guo, J. U., Su, Y., Zhong, C., Ming, G. L., & Song, H. (2011). Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell, 145(3), 423–434.
Lemaitre, J. M., Danis, E., Pasero, P., Vassetzky, Y., & Méchali, M. (2005). Mitotic remodeling of the replicon and chromosome structure. Cell, 123(5), 787–801.
Miyamoto, K., Nagai, K., Kitamura, N., Nishikawa, T., Ikegami, H., Binh, N. T., et al. (2011). Identification and characterization of an oocyte factor required for development of porcine nuclear transfer embryos. Proceedings of the National Academy of Sciences of the United States of America, 108(17), 7040–7045.
Egli, D., Birkhoff, G., & Eggan, K. (2008). Mediators of reprogramming: transcription factors and transitions through mitosis. Nature Reviews Molecular Cell Biology, 9(7), 505–516.
Inoue, K., Kohda, T., Sugimoto, M., Sado, T., Ogonuki, N., Matoba, S., et al. (2010). Impeding Xist expression from the active X chromosome improves mouse somatic cell nuclear transfer. Science, 330(6003), 496–499.
Wen, B., Wu, H., Shinkai, Y., Irizarry, R. A., & Feinberg, A. P. (2009). Large histone H3 lysine 9 dimethylated chromatin blocks distinguish differentiated from embryonic stem cells. Nature Genetics, 41(2), 246–250.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., et al. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872.
Tamada, H., Van Thuan, N., Reed, P., Nelson, D., Katoku-Kikyo, N., Wudel, J., et al. (2006). Chromatin decondensation and nuclear reprogramming by nucleoplasmin. Molecular and Cellular Biology, 26(4), 1259–1271.
Gaspar-Maia, A., Alajem, A., Polesso, F., Sridharan, R., Mason, M. J., Heidersbach, A., et al. (2009). Chd1 regulates open chromatin and pluripotency of embryonic stem cells. Nature, 460(7257), 863–868.
Singhal, N., Graumann, J., Wu, G., Araúzo-Bravo, M. J., Han, D. W., Greber, B., et al. (2010). Chromatin-Remodeling Components of the BAF complex facilitate reprogramming. Cell, 141(6), 943–955.
Liang, G., He, J., & Zhang, Y. (2012). Kdm2b promotes induced pluripotent stem cell generation by facilitating gene activation early in reprogramming. Nature Cell Biology, 14(5), 457–466.
Epsztejn-Litman, S., Feldman, N., Abu-Remaileh, M., Shufaro, Y., Gerson, A., Ueda, J., et al. (2008). De novo DNA methylation promoted by G9a prevents reprogramming of embryonically silenced genes. Nature Structural and Molecular Biology, 15(11), 1176–1183.
Wossidlo, M., Nakamura, T., Lepikhov, K., Marques, C. J., Zakhartchenko, V., Boiani, M., et al. (2011). 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nature Communications, 2, 241.
Gu, T. P., Guo, F., Yang, H., Wu, H. P., Xu, G. F., Liu, W., et al. (2011). The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature, 477(7366), 606–610.
Pasque, V., Gillich, A., Garrett, N., & Gurdon, J. B. (2011). Histone variant macroH2A confers resistance to nuclear reprogramming. EMBO Journal, 30(12), 2373–2387.
Angelov, D., Molla, A., Perche, P. Y., Hans, F., Côté, J., Khochbin, S., et al. (2003). The histone variant macroH2A interferes with transcription factor binding and SWI/SNF nucleosome remodeling. Molecular Cell, 11(4), 1033–1041.
Shi, Y., Desponts, C., Do, J. T., Hahm, H. S., Schöler, H. R., & Ding, S. (2008). Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell, 3(5), 568–574.
Li, S. C., Jin, Y., Loudon, W. G., Song, Y., Ma, Z., Weiner, L. P., et al. (2011). Increase developmental plasticity of human keratinocytes with gene suppression. Proceedings of the National Academy of Sciences of the United States of America, 108(31), 12793–12798.
Kawamura, T., Suzuki, J., Wang, Y. V., Menendez, S., Morera, L. B., Raya, A., et al. (2009). Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature, 460(7259), 1140–1144.
Tanaka, M., Hennebold, J. D., Macfarlane, J., & Adashi, E. Y. (2001). A mammalian oocyte-specific linker histone gene H1oo: homology with the genes for the oocyte-specific cleavage stage histone (cs-H1) of sea urchin and the B4/H1M histone of the frog. Development, 128(5), 655–664.
Onder, T. T., Kara, N., Cherry, A., Sinha, A. U., Zhu, N., Bernt, K. M., et al. (2012). Chromatin-modifying enzymes as modulators of reprogramming. Nature, 483(7391), 598–602.
Huangfu, D., Maehr, R., Guo, W., Eijkelenboom, A., Snitow, M., Chen, A. E., et al. (2008). Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nature Biotechnology, 26(7), 795–797.
Lluis, F., Ombrato, L., Pedone, E., Pepe, S., Merrill, B. J., & Cosma, M. P. (2011). T-cell factor 3 (Tcf3) deletion increases somatic cell reprogramming by inducing epigenome modifications. Proceedings of the National Academy of Sciences of the United States of America, 108(29), 11912–11917.
Ang, Y. S., Tsai, S. Y., Lee, D. F., Monk, J., Su, J., Ratnakumar, K., et al. (2011). Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell, 145(2), 183–197.
Xu, N., Papagiannakopoulos, T., Pan, G., Thomson, J. A., & Kosik, K. S. (2009). MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell, 137(4), 647–658.
Li, J., Ishii, T., Feinstein, P., & Mombaerts, P. (2004). Odorant receptor gene choice is reset by nuclear transfer from mouse olfactory sensory neurons. Nature, 428(6981), 393–399.
Eggan, K., Baldwin, K., Tackett, M., Osborne, J., Gogos, J., Chess, A., et al. (2004). Mice cloned from olfactory sensory neurons. Nature, 428(6978), 44–49.
Hochedlinger, K., & Jaenisch, R. (2002). Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature, 415(6875), 1035–1038.
Inoue, K., Wakao, H., Ogonuki, N., Miki, H., Seino, K., Nambu-Wakao, R., et al. (2005). Generation of cloned mice by direct nuclear transfer from natural killer T cells. Current Biology, 15(12), 1114–1118.
Wakayama, S., Ohta, H., Kishigami, S., Thuan, N. V., Hikichi, T., Mizutani, E., et al. (2005). Establishment of male and female nuclear transfer embryonic stem cell lines from different mouse strains and tissues. Biology of Reproduction, 72(4), 932–936.
Blelloch, R. H., Hochedlinger, K., Yamada, Y., Brennan, C., Kim, M., Mintz, B., et al. (2004). Nuclear cloning of embryonal carcinoma cells. Proceedings of the National Academy of Sciences of the United States of America, 101(39), 13985–13990.
Blelloch, R., Wang, Z., Meissner, A., Pollard, S., Smith, A., & Jaenisch, R. (2006). Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells, 24(9), 2007–2013.
Blau, H. M., Pavlath, G. K., Hardeman, E. C., Chiu, C. P., Silberstein, L., Webster, S. G., et al. (1985). Plasticity of the differentiated state. Science, 230(4727), 758–766.
Eminli, S., Foudi, A., Stadtfeld, M., Maherali, N., Ahfeldt, T., Mostoslavsky, G., et al. (2009). Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nature Genetics, 41(9), 968–976.
Acknowledgments
The author is grateful to Prof. Mansoureh Movahedin of Tarbiat Modarres University for her kind encouragement of this work and her useful comments on the manuscript.
Conflict of interest statement
The author indicates no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anjamrooz, S.H. The Cellular Memory Disc of Reprogrammed Cells. Stem Cell Rev and Rep 9, 190–209 (2013). https://doi.org/10.1007/s12015-013-9429-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-013-9429-4