Abstract
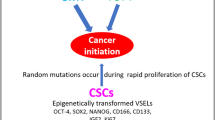
The morphology of several tumors mimics developmentally early tissues; tumors often express early developmental markers characteristic for the germ line lineage. Recently, our group identified a population of very small stem cells (SCs) in murine bone marrow (BM) and other adult organs that express several markers characteristic for epiblast/germ line-derived SCs. We named these rare cells “Very Small Embryonic/Epiblast-like Stem Cells (VSELs).” We hypothesized that these cells that express both epiblast and germ line markers are deposited during early gastrulation in developing tissues and organs and play an important role in the turnover of tissue-committed (TC) SCs. To support this, we envision that the germ line is not only the origin of SCs, but also remains as a scaffold or back-up for the SC compartment in adult life. Furthermore, we noticed that VSELs are protected from uncontrolled proliferation and teratoma formation by a unique DNA methylation pattern in some developmentally crucial imprinted genes, which show hypomethylation or erasure of imprints in paternally methylated genes and hypermethylation of imprints in the maternally methylated. In pathological situations, however, we hypothesize that VSELs could be involved in the development of several malignancies. Therefore, potential involvement of VSELs in cancerogenesis could support century-old concepts of embryonic rest- or germ line-origin hypotheses of cancer development. However, we are aware that this working hypothesis requires further direct experimental confirmation.




Similar content being viewed by others
Abbreviations
- BM:
-
Bone marrow
- BMMNC:
-
Bone marrow mononuclear cell
- C/T:
-
Cancer testis
- DMR:
-
Differently methylated region
- dpc:
-
Days post-conception
- EGC:
-
Embryonic germ cell
- ESC:
-
Embryonic stem cell
- FACS:
-
Fluorescence-activated cell sorting
- FC:
-
Flow cytometry
- GC:
-
Germ cell
- HSC:
-
Hematopoietic stem cell
- ICM:
-
Inner cell mass
- Igf1R:
-
Insulin-like growth factor 1 receptor
- Igf2:
-
Insulin-like growth factor 2
- Igf2R:
-
Igf2 receptor
- ISS:
-
ImageStream system
- PGC:
-
Primordial germ cell
- PSC:
-
Pluripotent stem cell
- Rasgrf1:
-
Ras protein-specific guanine nucleotide-releasing factor 1
- SC:
-
Stem cell
- SSEA-1:
-
Stage-specific embryonic antigen-1
- TCSC:
-
Tissue-committed stem cell
- VSEL:
-
Very small embryonic/epiblast-like stem cell
References
Kim, C. F., Jackson, E. L., Woolfenden, A. E., Lawrence, S., Babar, I., Vogel, S., et al. (2005). Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell, 121, 823–835.
Singh, S. K., Hawkins, C., Clarke, I. D., Squire, J. A., Bayani, J., Hide, T., et al. (2004). Identification of human brain tumour initiating cells. Nature, 432, 396–401.
Bonnet, D., & Dick, J. E. (1997). Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Medicine, 3, 730–737.
Reya, T., Morrison, S. J., Clarke, M. F., & Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414, 105–111.
Houghton, J., Stoicov, C., Nomura, S., Rogers, A. B., Carlson, J., Li, H., et al. (2004). Gastric cancer originating from bone marrow-derived cells. Science, 306, 1568–1571.
Fang, D., Nguyen, T. K., Leishear, K., Finko, R., Kulp, A. N., Hotz, S., et al. (2005). A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Research, 65, 9328–9337.
Welm, B., Behbod, F., Goodell, M. A., & Rosen, J. M. (2003). Isolation and characterization of functional mammary gland stem cells. Cell Proliferation, 36(Suppl 1), 17–32.
Boiani, M., & Scholer, H. R. (2005). Regulatory networks in embryo-derived pluripotent stem cells. Nature Reviews Molecular Cell Biology, 6, 872–884.
O’Farrell, P. H., Stumpff, J., & Su, T. T. (2004). Embryonic cleavage cycles: how is a mouse like a fly? Current Biology, 14, R35–R45.
Brons, I. G., Smithers, L. E., Trotter, M. W., Rugg-Gunn, P., Sun, B., & Chuva de Sousa Lopes, S. M. (2007). Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature, 448, 191–195.
Kucia, M., Reca, R., Campbell, F. R., Zuba-Surma, E., Majka, M., Ratajczak, J., et al. (2006). A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia, 20, 857–869.
Ratajczak, M. Z., Machalinski, B., Wojakowski, W., Ratajczak, J., & Kucia, M. (2007). A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia, 21, 860–867.
Ratajczak, M. Z., Zuba-Surma, E. K., Machalinski, B., Ratajczak, J., & Kucia, M. (2008). Very small embryonic-like (VSEL) stem cells: purification from adult organs, characterization, and biological significance. Stem Cells Review, 4, 89–99.
Zuba-Surma, E. K., Kucia, M., Wu, W., Klich, I., Lillard, J. W., Jr., Ratajczak, J., et al. (2008). Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies. Cytometry A, 73A, 1116–1127.
Shin, D. M., Zuba-Surma, E. K., Wu, W., Ratajczak, J., Wysoczynski, M., Ratajczak, M. Z., et al. (2009). Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells. Leukemia, 23, 2042–2051.
Beltrami, A. P., Cesselli, D., Bergamin, N., Marcon, P., Rigo, S., Puppato, E., et al. (2007). Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow). Blood, 110, 3438–3446.
Jiang, Y., Jahagirdar, B. N., Reinhardt, R. L., Schwartz, R. E., Keene, C. D., Ortiz-Gonzalez, X. R., et al. (2002). Pluripotency of mesenchymal stem cells derived from adult marrow. Nature, 418, 41–49.
D’Ippolito, G., Diabira, S., Howard, G. A., Menei, P., Roos, B. A., & Schiller, P. C. (2004). Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. Journal of Cell Science, 117, 2971–2981.
Pochampally, R. R., Smith, J. R., Ylostalo, J., & Prockop, D. J. (2004). Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood, 103, 1647–1652.
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284, 143–147.
Yu, H., Fang, D., Kumar, S. M., Li, L., Nguyen, T. K., Acs, G., et al. (2006). Isolation of a novel population of multipotent adult stem cells from human hair follicles. American Journal of Pathology, 168, 1879–1888.
Jones, R. J., Wagner, J. E., Celano, P., Zicha, M. S., & Sharkis, S. J. (1990). Separation of pluripotent haematopoietic stem cells from spleen colony-forming cells. Nature, 347, 188–189.
Donovan, P. J. (1998). The germ cell—the mother of all stem cells. International Journal of Developmental Biology, 42, 1043–1050.
Zwaka, T. P., & Thomson, J. A. (2005). A germ cell origin of embryonic stem cells? Development, 132, 227–233.
McLaren, A. (2003). Primordial germ cells in the mouse. Developmental Biology, 262, 1–15.
McLaren, A. (1992). Development of primordial germ cells in the mouse. Andrologia, 24, 243–247.
Yamanaka, Y., Ralston, A., Stephenson, R. O., & Rossant, J. (2006). Cell and molecular regulation of the mouse blastocyst. Developmental Dynamics, 235, 2301–2314.
De Felici, M., & McLaren, A. (1983). In vitro culture of mouse primordial germ cells. Experimental Cell Research, 144, 417–427.
Yamazaki, Y., Mann, M. R., Lee, S. S., Marh, J., McCarrey, J. R., Yanagimachi, R., et al. (2003). Reprogramming of primordial germ cells begins before migration into the genital ridge, making these cells inadequate donors for reproductive cloning. Proceedings of the National Academy of Sciences of the United States of America, 100, 12207–12212.
Lee, J., Inoue, K., Ono, R., Ogonuki, N., Kohda, T., Kaneko-Ishino, T., et al. (2002). Erasing genomic imprinting memory in mouse clone embryos produced from day 11.5 primordial germ cells. Development, 129, 1807–1817.
Mann, J. R. (2001). Imprinting in the germ line. Stem Cells, 19, 287–294.
Lees-Murdock, D. J., & Walsh, C. P. (2008). DNA methylation reprogramming in the germ line. Epigenetics, 3, 5–13.
Delaval, K., & Feil, R. (2004). Epigenetic regulation of mammalian genomic imprinting. Current Opinion in Genetics and Development, 14, 188–195.
Sasaki, H., Ishihara, K., & Kato, R. (2000). Mechanisms of Igf2/H19 imprinting: DNA methylation, chromatin and long-distance gene regulation. Journal of Biochemistry, 127, 711–715.
Reik, W. (2007). Stability and flexibility of epigenetic gene regulation in mammalian development. Nature, 447, 425–432.
Surani, M. A. (2001). Reprogramming of genome function through epigenetic inheritance. Nature, 414, 122–128.
Donovan, P. J. (1994). Growth factor regulation of mouse primordial germ cell development. Current Topics in Developmental Biology, 29, 189–225.
Matsui, Y., Zsebo, K., & Hogan, B. L. (1992). Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell, 70, 841–847.
Resnick, J. L., Ortiz, M., Keller, J. R., & Donovan, P. J. (1998). Role of fibroblast growth factors and their receptors in mouse primordial germ cell growth. Biology of Reproduction, 59, 1224–1229.
Shamblott, M. J., Axelman, J., Wang, S., Bugg, E. M., Littlefield, J. W., Donovan, P. J., et al. (1998). Derivation of pluripotent stem cells from cultured human primordial germ cells. Proceedings of the National Academy of Sciences of the United States of America, 95, 13726–13731.
Kono, T., Obata, Y., Wu, Q., Niwa, K., Ono, Y., Yamamoto, Y., et al. (2004). Birth of parthenogenetic mice that can develop to adulthood. Nature, 428, 860–864.
Kato, Y., Rideout, W. M., 3rd, Hilton, K., Barton, S. C., Tsunoda, Y., & Surani, M. A. (1999). Developmental potential of mouse primordial germ cells. Development, 126, 1823–1832.
Durcova-Hills, G., & Surani, A. (2008). Reprogramming primordial germ cells (PGC) to embryonic germ (EG) cells. Curr Protoc Stem Cell Biol, Chapter 1:Unit1A 3
Kaneda, A., Wang, C. J., Cheong, R., Timp, W., Onyango, P., Wen, B., et al. (2007). Enhanced sensitivity to IGF-II signaling links loss of imprinting of IGF2 to increased cell proliferation and tumor risk. Proceedings of the National Academy of Sciences of the United States of America, 104, 20926–20931.
Hartmann, W., Koch, A., Brune, H., Waha, A., Schuller, U., Dani, I., et al. (2005). Insulin-like growth factor II is involved in the proliferation control of medulloblastoma and its cerebellar precursor cells. American Journal of Pathology, 166, 1153–1162.
Hao, Y., Crenshaw, T., Moulton, T., Newcomb, E., & Tycko, B. (1993). Tumour-suppressor activity of H19 RNA. Nature, 365, 764–767.
Pollak, M. (2008). Insulin and insulin-like growth factor signalling in neoplasia. Nature Reviews Cancer, 8, 915–928.
Font de Mora, J., Esteban, L. M., Burks, D. J., Nunez, A., Garces, C., Garcia-Barrado, M. J., et al. (2003). Ras-GRF1 signaling is required for normal beta-cell development and glucose homeostasis. EMBO Journal, 22, 3039–3049.
Oosterhuis, J. W., & Looijenga, L. H. (2005). Testicular germ–cell tumours in a broader perspective. Nature Reviews Cancer, 5, 210–222.
Macchiarini, P., & Ostertag, H. (2004). Uncommon primary mediastinal tumours. Lancet Oncology, 5, 107–118.
Andrews, P. W., Matin, M. M., Bahrami, A. R., Damjanov, I., Gokhale, P., & Draper, J. S. (2005). Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochemical Society Transactions, 33, 1526–1530.
Sigalotti, L., Covre, A., Zabierowski, S., Himes, B., Colizzi, F., Natali, P. G., et al. (2008). Cancer testis antigens in human melanoma stem cells: expression, distribution, and methylation status. Journal of Cellular Physiology, 215, 287–291.
Simpson, A. J., Caballero, O. L., Jungbluth, A., Chen, Y. T., & Old, L. J. (2005). Cancer/testis antigens, gametogenesis and cancer. Nature Reviews Cancer, 5, 615–625.
Ratajczak, M. Z., Shin, D. M., & Kucia, M. (2009). Very small embryonic/epiblast-like stem cells: a missing link to support the germ line hypothesis of cancer development? American Journal of Pathology, 174, 1985–1992.
Hotakainen, K., Ljungberg, B., Haglund, C., Nordling, S., Paju, A., & Stenman, U. H. (2003). Expression of the free beta-subunit of human chorionic gonadotropin in renal cell carcinoma: prognostic study on tissue and serum. International Journal of Cancer, 104, 631–635.
Cheng, L. (2004). Establishing a germ cell origin for metastatic tumors using OCT4 immunohistochemistry. Cancer, 101, 2006–2010.
Barr, F. G. (1997). Molecular genetics and pathogenesis of rhabdomyosarcoma. Journal of Pediatric Hematology/Oncology, 19, 483–491.
Liu, C., Chen, Z., Chen, Z., Zhang, T., & Lu, Y. (2006). Multiple tumor types may originate from bone marrow-derived cells. Neoplasia, 8, 716–724.
Hernando, E. (2008). Cancer. Aneuploidy advantages? Science, 322, 692–693.
Acknowledgments
This work is supported by NIH grants R01 CA106281-01 and R01 DK074720 and the Stella and Henry Hoenig Endowment to MZR NIH grant P20RR018733 from the National Center for Research Resources to MK and European Union structural funds, Innovative Economy Operational Program POIG. 01.01.02-00-109/09-00.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ratajczak, M.Z., Shin, DM., Liu, R. et al. Epiblast/Germ Line Hypothesis of Cancer Development Revisited: Lesson from the Presence of Oct-4+ Cells in Adult Tissues. Stem Cell Rev and Rep 6, 307–316 (2010). https://doi.org/10.1007/s12015-010-9143-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-010-9143-4