Abstract
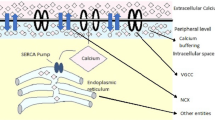
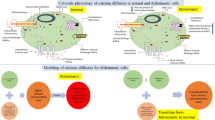
Free Calcium ions in the cytosol are essential for many physiological and physical functions. The free calcium ions are commonly regarded as a second messenger, are an essential part of brain communication. Numerous physiological activities, such as calcium buffering and calcium ion channel flow, etc. influence the cytosolic calcium concentration. In light of the above, the primary goal of this study is to develop a model of calcium distribution in neuron cells when a Voltage-Gated Calcium Channel and Sodium Calcium Exchanger are present. As we know, decreased buffer levels and increased calcium activity in the Voltage-Gated Calcium Channel and Sodium Calcium Exchanger lead to Alzheimer’s disease. Due to these changes, the calcium diffusion in that location becomes disrupted and impacted by Alzheimer’s disease. The model has been constructed by considering key factors like buffers and ER fluxes when Voltage-Gated Calcium Channels and Sodium Calcium Exchangers are present. Based on the physiological conditions of the parameters, appropriate boundary conditions have been constructed in the fuzzy environment. This model is considered a fuzzy boundary value problem with the source term and initial boundary conditions are modeled by triangular fuzzy functions. In this, paper we observed the approximate solution of the mathematical model which was investigated by the fuzzy undetermined coefficient method. The solution has been performed through MATLAB and numerical results have been computed using simulation. The observation made that the proper operation of the Voltage-Gated Calcium Channel and Sodium Calcium Exchanger is critical for maintaining the delicate equilibrium of calcium ions, which regulates vital cellular activities. Dysregulation of Voltage-Gated Calcium Channel and Sodium Calcium Exchanger activity has been linked to neurodegenerative illnesses like Alzheimer’s disease.










Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Korol’, T. Y., Korol’, S. V., Kostyuk, E. P., & Kostyuk, P. G. (2008). Disruption of calcium homeostasis in Alzheimer’s disease. Neirofiziologiya/Neurophysiology 40, 385–392.
Rajakulendran, S., & Hanna, M. G. (2016). The role of calcium channels in epilepsy. Cold Spring Harbor Perspectives in Medicine, 6(1). https://doi.org/10.1101/cshperspect.a022723.
Turkington C. (2010). The encyclopedia of Alzheimer’s disease (second.). Facts on file: an imprint of Infobase Publishing.
Magi, S., Castaldo, P., MacRi, M. L., Maiolino, M., Matteucci, A., Bastioli, G., … Lariccia, V. (2016). Intracellular calcium dysregulation: Implications for Alzheimer’s disease. BioMed Research International. Hindawi Limited. https://doi.org/10.1155/2016/6701324.
Khachaturian, Z. S. (1994). Calcium hypothesis of Alzheimer’s disease and brain aging a. Annals of the New York Academy of Sciences, 747(1), 1–11. https://doi.org/10.1111/j.1749-6632.1994.tb44398.x.
Rajagopal, S., & Ponnusamy, M. (2017). Calcium Signaling: From Physiology to Diseases. Singapore: Singapore: Springer. https://doi.org/10.1007/978-981-10-5160-9.
Khachaturian, Z. S. (1989). Introduction and overview. Annals of the New York Academy of Sciences, 568(1), 1–4. https://doi.org/10.1111/j.1749-6632.1989.tb12485.x.
Bezprozvanny, I. (2009). Calcium signaling and neurodegenerative diseases. Trends in Molecular Medicine, 15(3), 89–100. https://doi.org/10.1016/j.molmed.2009.01.001.
Dave, D. D., & Jha, B. K. (2018). Delineation of calcium diffusion In Alzheimeric brain. Journal of Mechanics in Medicine and Biology, 18(03), 1850028. https://doi.org/10.1142/S0219519418500288.
Dave, D. D., & Jha, B. K. (2018). Analytically depicting the calcium diffusion for Alzheimer’s affected cell. International Journal of Biomathematics, 11(7). https://doi.org/10.1142/S1793524518500882.
Dave, D. D., & Jha, B. K. (2020). 3D mathematical modeling of calcium signaling in Alzheimer’s disease. Network Modeling Analysis in Health Informatics and Bioinformatics, 9(1), 1. https://doi.org/10.1007/s13721-019-0207-3.
Jha, A., Adlakha, N., & Jha, B. K. (2016). Finite element model to study effect of Na+−Ca2+ exchangers and source geometry on calcium dynamics in a neuron cell. Journal of Mechanics in Medicine and Biology, 16(02), 1650018. https://doi.org/10.1142/S0219519416500184.
Jha, A., & Adlakha, N. (2014). Finite element model to study the effect of exogenous buffer on calcium dynamics in dendritic spines. International Journal of Modeling, Simulation, and Scientific Computing, 05(02), 1350027. https://doi.org/10.1142/S179396231350027X.
Panday, S., & Pardasani, K. R. (2013). Finite element model to study effect of advection diffusion and Na+/Ca2+ exchanger on Ca2+ distribution in oocytes. Journal of Medical Imaging and Health Informatics, 3(3), 374–379. https://doi.org/10.1166/jmihi.2013.1184.
Naik, P. A., & Pardasani, K. R. (2019). Three-dimensional finite element model to study effect of RyR calcium channel, ER Leak and SERCA pump on calcium distribution in oocyte cell. International Journal of Computational Methods, 16(01), 1850091. https://doi.org/10.1142/S0219876218500913.
Naik, P. A., & Pardasani, K. R. (2016). Finite element model to study calcium distribution in oocytes involving voltage gated Ca 2+ channel, ryanodine receptor and buffers. Alexandria Journal of Medicine, 52(1), 43–49. https://doi.org/10.1016/j.ajme.2015.02.002.
Kumar, H., Naik, P. A., & Pardasani, K. R. (2018). Finite element model to study calcium distribution in T lymphocyte involving buffers and ryanodine receptors. Proceedings of the National Academy of Sciences, India Section A: Physical Sciences, 88(4), 585–590. https://doi.org/10.1007/s40010-017-0380-7.
Naik, P. A., & Pardasani, K. R. (2017). Three-dimensional finite element model to study calcium distribution in oocytes. Network Modeling Analysis in Health Informatics and Bioinformatics, 6(1), 16. https://doi.org/10.1007/s13721-017-0158-5.
Singh, T., & Adlakha, N. (2023). Numerical investigations and simulation of calcium distribution in the alpha-cell. Bulletin of Biomathematics, https://doi.org/10.59292/bulletinbiomath.2023003.
Naik, P. A. (2020). Modeling the mechanics of calcium regulation in T lymphocyte: a finite element method approach. International Journal of Biomathematics, 13(05), 2050038. https://doi.org/10.1142/S1793524520500382.
Pawar, A., & Pardasani, K. R. (2023). Fractional-order reaction–diffusion model to study the dysregulatory impacts of superdiffusion and memory on neuronal calcium and IP3 dynamics. The European Physical Journal Plus, 138(9), 780. https://doi.org/10.1140/epjp/s13360-023-04410-6.
Pawar, A., & Raj Pardasani, K. (2022). Effects of disorders in interdependent calcium and IP3 dynamics on nitric oxide production in a neuron cell. The European Physical Journal Plus, 137(5), 543. https://doi.org/10.1140/epjp/s13360-022-02743-2.
Pawar, A., & Pardasani, K. R. (2024). Nonlinear system dynamics of calcium and nitric oxide due to cell memory and superdiffusion in neurons. Communications in Theoretical Physics, https://doi.org/10.1088/1572-9494/ad35b4
Jethanandani, H., Jha, B. K., & Ubale, M. (2023). Bifurcation analysis of calcium dynamics in nerve cell. The European Physical Journal Plus, 138(12), 1159. https://doi.org/10.1140/epjp/s13360-023-04699-3.
Jethanandani, H., Jha, B. K., & UBALE, M. (2023). The role of calcium dynamics with amyloid beta on neuron-astrocyte coupling. Mathematical Modelling and Numerical Simulation with Applications, 3(4), 376–390. https://doi.org/10.53391/mmnsa.1398320.
Manhas, N., Camara, A. K. S., & Dash, R. K. (2018). Modeling mechanisms of cardiac L-Type Ca2+ channel regulation: interactions of voltage, Ca2+, and isoflurane. Biophysical Journal, 114(3), 304a. https://doi.org/10.1016/j.bpj.2017.11.1728.
Jha, B. K., & Dave, D. D. (2020). Approximation of calcium diffusion in Alzheimeric cell. Journal of Multiscale Modelling, 11(02). https://doi.org/10.1142/S1756973720500018.
Kumar Jha, B., Adlakha, N., & Mehta, M. N. (2012). Analytic solution of two dimensional advection diffusion equation arising in cytosolic calcium concentration distribution. International Mathematical Forum, 7, 135–144.
Joshi, H., & Jha, B. K. (2023). 2D memory-based mathematical analysis for the combined impact of calcium influx and efflux on nerve cells. Computers & Mathematics with Applications, 134, 33–44. https://doi.org/10.1016/j.camwa.2022.12.016.
Joshi, H., & Jha, B. K. (2022). 2D dynamic analysis of the disturbances in the calcium neuronal model and its implications in neurodegenerative disease. Cognitive Neurodynamics, https://doi.org/10.1007/s11571-022-09903-1.
Kothiya, A., & Adlakha, N. (2023). Simulation of biochemical dynamics of Ca2+ and PLC in fibroblast cell. Journal of Bioenergetics and Biomembranes, 55(4), 267–287.
Jagpat, Y., & Adlakha, N. (2023). Numerical model of hepatic glycogen phosphorylase regulation by nonlinear interdependent dynamics of calcium and IP3. The European Physical Journal Plus. https://doi.org/10.1140/epjp/s13360-023-03961-y.
Pawar, A., & Pardasani, K. R. (2023). Computational model of calcium dynamics-dependent dopamine regulation and dysregulation in a dopaminergic neuron cell. The European Physical Journal Plus, 138(1), 30. https://doi.org/10.1140/epjp/s13360-023-03691-1.
Joshi, H., & Yavuz, M. (2024). Numerical analysis of compound biochemical calcium oscillations process in hepatocyte cells. Advanced Biology, https://doi.org/10.1002/adbi.202300647.
Pawar, A., & Pardasani, K. R. (2024). Modelling cross talk in the spatiotemporal system dynamics of calcium, IP3 and nitric oxide in neuron cells. Cell Biochemistry and Biophysics, https://doi.org/10.1007/s12013-024-01229-5.
Kandel, A., & Byatt, W. J. (1978). Fuzzy sets, fuzzy algebra, and fuzzy statistics. Proceedings of the IEEE, 66(12), 1619–1639. https://doi.org/10.1109/PROC.1978.11171.
Kaleva, O. (1987). Fuzzy differential equations. Fuzzy Sets and Systems, 24(3), 301–317. https://doi.org/10.1016/0165-0114(87)90029-7.
Kaleva, O. (1990). The Cauchy problem for fuzzy differential equations. Fuzzy Sets and Systems, 35(3), 389–396. https://doi.org/10.1016/0165-0114(90)90010-4.
Bede, B., & Gal, S. G. (2005). Generalizations of the differentiability of fuzzy-number-valued functions with applications to fuzzy differential equations. Fuzzy Sets and Systems, 151(3), 581–599. https://doi.org/10.1016/j.fss.2004.08.001.
O’Regan, D., Lakshmikantham, V., & Nieto, J. J. (2003). Initial and boundary value problems for fuzzy differential equations. Nonlinear Analysis, Theory, Methods and Applications, 54(3), 405–415. https://doi.org/10.1016/S0362-546X(03)00097-X.
Allahviranloo, T., Ahmady, E., & Ahmady, N. (2008). Nth-order fuzzy linear differential equations. Information Sciences, 178(5), 1309–1324. https://doi.org/10.1016/j.ins.2007.10.013.
Bhattacharyya, R., & Jha, B. K. (2024). Analyzing fuzzy boundary value problems: a study on the influence of mitochondria and ER fluxes on calcium ions in neuron cells. Journal of Bioenergetics and Biomembranes, 56(1), 15–29. https://doi.org/10.1007/s10863-023-09994-3.
Bede, B. (2006). A note on “two-point boundary value problems associated with non-linear fuzzy differential equations. Fuzzy Sets and Systems, 157(7), 986–989. https://doi.org/10.1016/j.fss.2005.09.006.
Chalco-Cano, Y., & Román-Flores, H. (2008). On new solutions of fuzzy differential equations. Chaos, Solitons and Fractals, 38(1), 112–119. https://doi.org/10.1016/j.chaos.2006.10.043.
Bede, B., & Stefanini, L. (2011). Solution of Fuzzy Differential Equations with generalized differentiability using LU-parametric representation. In Proceedings of the 7th Conference of the European Society for Fuzzy Logic and Technology (EUSFLAT-2011). Paris, France: Atlantis Press https://doi.org/10.2991/eusflat.2011.106.
Gomes, L. T., & Barros, L. C. (2015). A note on the generalized difference and the generalized differentiability. Fuzzy Sets and Systems, 280, 142–145. https://doi.org/10.1016/j.fss.2015.02.015.
Stefanini, L. (2008). A generalization of Hukuhara difference. Advances in Soft Computing, 48, 203–210. https://doi.org/10.1007/978-3-540-85027-4_25.
Rohatgi, V. K., Ben-Israel, A., & Greville, T. N. E. (1976). Generalized Inverses: Theory and Applications. International Statistical Review / Revue Internationale de Statistique, 44(2), 301. https://doi.org/10.2307/1403291.
Schwaller, B. (2020). Cytosolic Ca2+ buffers are inherently Ca2+ signal modulators. Cold Spring Harbor Perspectives in Biology, 12(1). https://doi.org/10.1101/cshperspect.a035543.
Smith, G. D. (1996). Analytical steady-state solution to the rapid buffering approximation near an open Ca2+ channel. Biophysical Journal, 71(6), 3064–3072. https://doi.org/10.1016/S0006-3495(96)79500-0.
Smith, G. D., Dai, L., Miura, R. M., & Sherman, A. (2001). Asymptotic analysis of buffered calcium diffusion near a point source. SIAM Journal on Applied Mathematics, 61(5). https://doi.org/10.1137/S0036139900368996.
Jha, B. K., Adlakha, N., & Mehta, M. N. (2013). Two-dimensional finite element model to study calcium distribution in astrocytes in presence of VGCC and excess buffer. International Journal of Modeling, Simulation, and Scientific Computing, 4(2). https://doi.org/10.1142/S1793962312500304.
Antman, S. S., Marsden, J. E., Sirovich, L., Biology, M., Glass, L., Murray, J. D., & Kohn, R. V. (2002). Interdisciplinary applied mathematics. Mathematical Biology I. An Introduction, Third Edition, Verlag, New York, Berlin, Heidelberg: Springer.
De Young, G. W., & Keizer, J. (1992). A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proceedings of the National Academy of Sciences, 89(20), 9895–9899. https://doi.org/10.1073/pnas.89.20.9895.
Jha, B. K., Adlakha, N., & Mehta, M. N. (2011). Finite volume model to study the effect of ER flux on cytosolic calcium distribution in astrocytes. Journal of Computing, 3(1).
Yagami, T., Kohma, H., & Yamamoto, Y. (2012). L-type voltage-dependent calcium channels as therapeutic targets for neurodegenerative diseases. Current Medicinal Chemistry, 19(28), 4816–4827. https://doi.org/10.2174/092986712803341430.
James P. K., & James S. (2009). Mathematical physiology (2nd, illustrated ed., Vol. volume 8). Springer, 2009.
Colvin, R. A., Davis, N., Wu, A., Murphy, C. A., & Levengood, J. (1994). Studies of the mechanism underlying increased Na+/Ca2+ exchange activity in Alzheimer’s disease brain. Brain Research, 665(2), 192–200. https://doi.org/10.1016/0006-8993(94)91338-2.
Tewari, V., Raj Pardasani, K., Tewari, S., & Pardasani, K. (2011). A model to study the effect of excess buffers and Na+ ions on Ca2+ diffusion in neuron cell, https://doi.org/10.5281/zenodo.1054988.
Annunziato, L., Pignataro, G., & Di Renzo, G. F. (2004). Pharmacology of brain Na + /Ca 2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacological Reviews, 56(4), 633–654. https://doi.org/10.1124/pr.56.4.5.
Fujioka, Y., Hiroe, K., & Matsuoka, S. (2000). Regulation kinetics of Na + ‐Ca 2+ exchange current in guinea‐pig ventricular myocytes. The Journal of Physiology, 529(3), 611–623. https://doi.org/10.1111/j.1469-7793.2000.00611.x.
Dave, D. D., & Jha, B. K. (2021). 2D finite element estimation of calcium diffusion in Alzheimer’s affected neuron. Network Modeling Analysis in Health Informatics and Bioinformatics, 10(1). https://doi.org/10.1007/s13721-021-00322-6.
Larry S., Darwin B., Floyd E. B., du Sascha L., Anirvan G., Nicholas C. S., Larry R. S. Fundamental Neuroscience 3rd ed. (2008).
Acknowledgements
The author is highly thankful to the SHODH scheme, the education department, Government of Gujarat, India for financial support for carrying out this research work.
Author information
Authors and Affiliations
Contributions
Both the first and second authors contributed equally to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have never had any known competing financial interests or personal ties that may seem to have influenced the work revealed in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jha, B.K., Bhattacharyya, R. A Comprehensive Fuzzy Model for Understanding Neuronal Calcium Distribution in Presence of VGCC, Na+/Ca2+ Exchanger, Buffer, and ER Fluxes. Cell Biochem Biophys (2024). https://doi.org/10.1007/s12013-024-01291-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s12013-024-01291-z