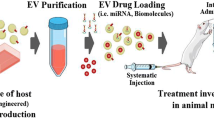
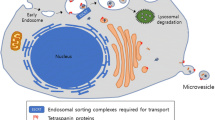
Abstract
In normal and pathophysiological conditions our cells secrete vesicular bodies known as extracellular particles. Extracellular vesicles are lipid-bound extracellular particles. A majority of these extracellular vesicles are linked to cell-to-cell communication. Brain consists of tightly packed neural cells. Neural cell releases extracellular vesicles in cerebrospinal fluid. Extracellular vesicle mediated crosstalk maintains neural homeostasis in the central nervous system via transferring cargos between neural cells. In neurodegenerative diseases, small extracellular vesicle transfer misfolded proteins to healthy cells in the neural microenvironment. They can also cross blood-brain barrier (BBB) and stimulate peripheral immune response inside central nervous system. In today’s world different approaches employ extracellular vesicle in various therapeutics. This review gives a brief knowledge about the biological relevance of extracellular vesicles in the central nervous system and relevant advances in the translational application of EV in brain disorders.
Graphical Abstract






Similar content being viewed by others
Abbreviations
- EVs:
-
Extracellular Vesicles
- BBB:
-
Blood Brain Barrier
- MVB:
-
Multivesicular Body
- MSC:
-
Mesenchymal Stem Cell
- NSC:
-
Neural Stem Cell
- ALS:
-
Amyotrophic lateral sclerosis
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- Aβ or Abeta:
-
Amyloid beta
- MS:
-
Multiple Sclerosis
- RT-qPCR:
-
Real-Time Quantitative Reverse Transcription PCR
- CD46:
-
Cluster of differentiation 46
- HIV-1:
-
Human immunodeficiency viruses type1
- SOD1:
-
Superoxide dismutase
- PrPSC :
-
Scrapie isoform of the prion protein
- CSF:
-
Cerebrospinal fluid
- CLN2:
-
Neuronal ceroidlipofuscinosis 2
- TPP1:
-
Tripeptidyl Peptidase 1
- m THPC:
-
Meta -tetra (hydroxyphenyl)chlorin
- HGF:
-
Hepatocyte growth factor
- Cre:
-
Cyclization recombinase
- Cys5:
-
Cyanines 5
- HEK293:
-
Human embryonic kidney 293 cells
- CNS:
-
Central Nervous System
- Wnt:
-
Wingless/Integrated
- IL1-β:
-
Interleukin-1β
- NFT:
-
Neurofibrillary tangles
- APP:
-
Amyloid Protein Precursor
- LBs:
-
Lewy Bodies
- MAPT:
-
Microtube-associated protein tau
- HD:
-
Huntington’s Disease
- mHTT:
-
mutant Huntingtin protein
- polyQ:
-
polyglutamine protein
- PRP:
-
Prion protein
- MDCK:
-
Madin – Darby Canine Kidney cell line
- Pgp:
-
P glycoprotein
- MDR:
-
Multiple Drug Resistance
References
Welsh, J. A., et al. (2024). Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. Journal of Extracellular Vesicles, 13, e12404. https://doi.org/10.1002/jev2.12404.
Anand, S., Samuel, M., & Mathivanan, S. (2021). Exomeres: a new member of extracellular vesicles family. Subcellular Biochemistry, 97, 89–97. https://doi.org/10.1007/978-3-030-67171-6_5.
Frühbeis, C., Fröhlich, D., & Krämer-Albers, E.-M. (2012). Emerging roles of exosomes in Neuron–Glia communication. Frontiers in Physiology, 3, 119. https://doi.org/10.3389/fphys.2012.00119.
van der Vos, K. E., Balaj, L., Skog, J., & Breakefield, X. O. (2011). Brain tumor microvesicles: Insights into intercellular communication in the nervous system. Cellular and Molecular Neurobiology, 31(6), 949–959. https://doi.org/10.1007/s10571-011-9697-y.
Di Daniele, A., Antonucci, Y., & Campello, S. (2022). Migrasomes, new vescicles as Hansel and Gretel white pebbles? Biology Direct, 17(1), 8. https://doi.org/10.1186/s13062-022-00321-1.
Zhang, X., Yao, L., & Meng, Y., et al. (2023). Migrasome: a new functional extracellular vesicle. Cell Death Discovery, 9, 381. https://doi.org/10.1038/s41420-023-01673-x.
Van Niel, G., Porto-Carreiro, I., Simoes, S., & Raposo, G. (2006). Exosomes: A common pathway for a specialized function. Journal of Biochemistry, 140, 13–21. https://doi.org/10.1093/jb/mvj128.
Géminard, C., de Gassart, A., Blanc, L., & Vidal, M. (2004). Degradation of AP2 during reticulocyte maturation enhances binding of Hsc70 and alix to a common siteon TfR for sorting into exosomes. Traffic., 5, 181–193. https://doi.org/10.1111/j.1600-0854.2004.0167.x.
Stuffers, S., Wegner, C. S., Stenmark, H., & Brech, A. (2009). Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic, 10, 925–937. https://doi.org/10.1111/j.1600-0854.2009.00920.x.
Crescitelli, R., Lässer, C., Szabó, T. G., Kittel, Á., Eldh, M., Dianzani, I., Buzás, E. I., & Lötvall, J. (2013). Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. Journal of Extracellular Vesicles, 2, 2. https://doi.org/10.3402/jev.v2i0.20677.
Sinha, A., Ignatchenko, V., Ignatchenko, A., Mejia-Guerrero, S., & Kislinger, T. (2014). In-depth proteomic analyses of ovarian cancer cell line exosomes reveals differential enrichment of functional categories compared to the NCI 60 proteome. Biochemical and Biophysical Research Communications, 445, 694–701. https://doi.org/10.1016/j.bbrc.2013.12.070.
Escrevente, C., Keller, S., Altevogt, P., & Costa, J. (2011). Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer, 11, 108. https://doi.org/10.1186/1471-2407-11-108.
Soto-Heredero, G., Baixauli, F., & Mittelbrunn, M. (2017). Interorganelle communication between mitochondria and the endolysosomal system. Frontiers in Cell Developmental Biology, 5, 95. https://doi.org/10.3389/fcell.2017.00095.
Frühbeis, C., Fröhlich, D., Kuo, W. P., Amphornrat, J., Thilemann, S., Saab, A. S., Kirchhoff, F., Möbius, W., Goebbels, S., Nave, K.-A., Schneider, A., Simons, M., Klugmann, M., Trotter, J. & Krämer-Albers, E.-M. (2013). Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte–neuron communication. PLoS Biology, 11(7), e1001604.
Saint-Pol, J., Gosselet, F., Duban-Deweer, S., Pottiez, G. & Karamanos, Y. (2020). Targeting and crossing the blood-brain barrier with extracellular vesicles. Cells, 9(4), 851.
Lachenal, G., Pernet-Gallay, K., Chivet, M., Hemming, F. J., Belly, A., Bodon, G., Blot, B., Haase, G., Goldberg, Y., & Sadoul, R. (2011). Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Molecular and Cellular Neuroscience, 46(2), 409–418. https://doi.org/10.1016/j.mcn.2010.11.004.
Deshpande, M., & Rodal, A. A. (2015). The crossroads of synaptic growth signaling, membrane traffic and neurological disease: insights from drosophila. Traffic, 17, 87–101. https://doi.org/10.1111/tra.12345.
Chivet, M., Javalet, C., Laulagnier, K., Blot, B., Hemming, F. J., & Sadoul, R. (2014). Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. Journal of Extracellular Vesicles, 3(1), 24722.
Dolcetti, E., Bruno, A., Guadalupi, L., Rizzo, F. R., Musella, A., Gentile, A., De Vito, F., Caioli, S., Bullitta, S., Fresegna, D., Vanni, V., Balletta, S., Sanna, K., Buttari, F., StampanoniBassi, M., Centonze, D. & Mandolesi, G. (2020). Emerging role of extracellular vesicles in the pathophysiology of multiple sclerosis. International Journal of Molecular Sciences, 21(19), 7336.
Serpe, C., Monaco, L., Relucenti, M., Iovino, L., Familiari, P., Scavizzi, F., Raspa, M., Familiari, G., Civiero, L., D’Agnano, I., Limatola, C. & Catalano, M. (2021). Microglia-derived small extracellular vesicles reduce glioma growth by modifying tumor cell metabolism and enhancing glutamate clearance through miR-124. Cells, 10(8), 2066.
Gharbi, T., Zhang, Z., & Yang, G.-Y. (2020). The function of astrocyte mediated extracellular vesicles in central nervous system diseases. Frontiers in Cell and Developmental Biology, 8, 568889. https://doi.org/10.3389/fcell.2020.568889.
Tajes, M., Ramos-Fernández, E., Weng-Jiang, X., Bosch-Morató, M., Guivernau, B., Eraso-Pichot, A., & Munoz, F. J. (2014). The blood-brain barrier: structure, function and therapeutic approaches to cross it. Molecular Membrane Biology, 31(5), 152–167.
Ridder, K., Keller, S., Dams, M., Rupp, A.-K., Schlaudraff, J., Del Turco, D., Starmann, J., Macas, J., Karpova, D., Devraj, K., Depboylu, C., Landfried, B., Arnold, B., Plate, K. H., Höglinger, G., Sültmann, H., Altevogt, P. & Momma, S. (2014). Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biology, 12(6), e1001874. https://doi.org/10.1371/journal.pbio.1001874.
Dickens, A. M., Tovar-y-Romo, L. B., Yoo, S.-W., Trout, A. L., Bae, M., Kanmogne, M., Megra, B., Williams, D. W., Witwer, K. W., Gacias, M., Tabatadze, N., Cole, R. N., Casaccia, P., Berman, J. W., Anthony, D. C. & Haughey, N. J. (2017). Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Science Signaling, 10(473), eaai7696. https://doi.org/10.1126/scisignal.aai7696.
Banks, W. A., Sharma, P., Bullock, K. M., Hansen, K. M., Ludwig, N. & Whiteside, T. L. (2020). Transport of extracellular vesicles across the blood-brain barrier: brain pharmacokinetics and effects of inflammation. International Journal of Molecular Sciences, 21(12), 4407.
Raub, T. J., & Audus, K. L. (1990). Adsorptive endocytosis and membrane recycling by cultured primary bovine brain microvessel endothelial cell monolayers. Journal of Cell Science, 97(1), 127–138. https://doi.org/10.1242/jcs.97.1.127.
Chen, C. C., Liu, L., Ma, F., Wong, C. W., Guo, X. E., Chacko, J. V., Farhoodi, H. P., Zhang, S. X., Zimak, J., Ségaliny, A., Riazifar, M., Pham, V., Digman, M. A., Pone, E. J., & Zhao, W. (2016). Elucidation of exosome migration across the Blood–Brain Barrier model in vitro. Cellular and Molecular Bioengineering, 9(4), 509–529. https://doi.org/10.1007/s12195-016-0458-3.
Ludwig, N., Yerneni, S. S., Razzo, B. M., & Whiteside, T. L. (2018). Exosomes from HNSCC promote angiogenesis through reprogramming of endothelial cells. Molecular Cancer Research, 16(11), 1798–1808. https://doi.org/10.1158/1541-7786.mcr-18-0358.
András, I. E., Leda, A., Contreras, M. G., Bertrand, L., Park, M., Skowronska, M., & Toborek, M. (2017). Extracellular vesicles of the blood-brain barrier: Role in the HIV-1 associated amyloid beta pathology. Molecular and Cellular Neuroscience, 79, 12–22. https://doi.org/10.1016/j.mcn.2016.12.006.
Bellingham, S. A., Guo, B., & Hill, A. F. (2012). Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Frontiers in Physiology, 3, 25554.
Howitt, J., & Hill, A. F. (2016). Exosomes in the pathology of neurodegenerative diseases. Journal of Biological Chemistry, 291(52), 26589–26597. https://doi.org/10.1074/jbc.r116.757955.
Malm, T., Loppi, S., & Kanninen, K. M. (2016). Exosomes in Alzheimer’s disease. Neurochemistry International, 97, 193–199.
Soliman, H. M., Ghonaim, G. A., Gharib, S. M., Chopra, H., Farag, A. K., Hassanin, M. H., Nagah, A., Emad-Eldin, M., Hashem, N. E., Yahya, G., Emam, S. E., Hassan, A. E. A. & Attia, M. S. (2021). Exosomes in Alzheimer’s disease: From being pathological players to potential diagnostics and therapeutics. International Journal of Molecular Sciences, 22(19), 10794.
Pinnell, J. R., Cui, M., & Tieu, K. (2021). Exosomes in Parkinson disease. Journal of Neurochemistry, 157(3), 413–428. https://doi.org/10.1111/jnc.15288.
Danzer, K. M., Kranich, L. R., Ruf, W. P., Cagsal-Getkin, O., Winslow, A. R., Zhu, L., Vanderburg, C. R. & McLean, P. J. (2012). Exosomal cell-to-cell transmission of alpha synuclein oligomers. Molecular Neurodegeneration, 7(1), 42.
Motani, A., Wang, Z., Conn, M., Siegler, K., Zhang, Y., Liu, Q., Johnstone, S., Xu, H., Thibault, S., Wang, Y., Connors, R., Le, H., Xu, G., Walker, N., Bei, S., & Coward, P. (2009). Identification and Characterization of a non-retinoid ligand for retinol-binding protein 4 which lowers serum retinol-binding protein 4 levels in vivo *. Journal of Biological Chemistry, 284(12), 7673–7680. https://doi.org/10.1074/jbc.
Cheng, L., Zhao, W., & Hill, A. F. (2018). Exosomes and their role in the intercellular trafficking of normal and disease associated prion proteins. Molecular Aspects of Medicine, 60, 62–68. https://doi.org/10.1016/j.mam.2017.11.011.
Cervenakova, L., Saá, P., Yakovleva, O., Vasilyeva, I., de Castro, J., Brown, P., & Dodd, R. (2016). Are prions transported by plasma exosomes? Transfusion and Apheresis Science, 55(1), 70–83. https://doi.org/10.1016/j.transci.2016.07.013.
Xiao, L., Hareendran, S., & Loh, Y. P. (2021). Function of exosomes in neurological disorders and brain tumors. Extracellular Vesicles and Circulating Nucleic Acids, 2, 55–79. https://doi.org/10.20517/evcna.2021.04.
Coulson, N. S., Buchanan, H., & Aubeeluck, A. (2007). Social support in cyberspace: A content analysis of communication within a Huntington’s disease online support group. Patient Education and Counseling, 68(2), 173–178. https://doi.org/10.1016/j.pec.2007.06.002.
Helder, D. I., Kaptein, A. A., van Kempen, G. M. J., van Houwelingen, J. C., & Roos, R. A. C. (2001). Impact of Huntington’s disease on quality of life. Movement Disorders, 16(2), 325–330. https://doi.org/10.1002/mds.1056.
McColgan, P., & Tabrizi, S. J. (2017). Huntington’s disease: a clinical review. European Journal of Neurology, 25(1), 24–34. https://doi.org/10.1111/ene.13413.
Costanzo, M., Abounit, S., Marzo, L., Danckaert, A., Chamoun, Z., Roux, P., & Zurzolo, C. (2013). Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. Journal of Cell Science, 126, 2685–3678.
Zhang, X., Abels, E. R., Redzic, J. S., Margulis, J., Finkbeiner, S., & Breakefield, X. O. (2016). Potential transfer of polyglutamine and CAG-repeat RNA in extracellular vesicles in Huntington’s disease: background and evaluation in cell culture. Cellular and Molecular Neurobiology, 36(3), 459–470. https://doi.org/10.1007/s10571-016-0350-7.
Bartel, D. P. (2004). MicroRNAs. Cell, 116(2), 281–297. https://doi.org/10.1016/s0092-8674(04)00045-5.
Leidinger, P., Backes, C., Deutscher, S., Schmitt, K., Mueller, S. C., Frese, K., Haas, J., Ruprecht, K., Paul, F., Stähler, C., Lang, C. J., Meder, B., Bartfai, T., Meese, E., & Keller, A. 2013). A blood based 12-miRNA signature of Alzheimer disease patients. Genome Biology, 14(7), R78.
Hoss, A. G., Labadorf, A., Beach, T. G., Latourelle, J. C. & Myers, R. H. (2016). microRNA Profiles in Parkinsona’s disease prefrontal cortex. Frontiers in Aging Neuroscience, 8, 36–44. https://doi.org/10.3389/fnagi.2016.00036.
Si, Y., Cui, X., Crossman, D. K., Hao, J., Kazamel, M., Kwon, Y., & King, P. H. (2018). Muscle microRNA signatures as biomarkers of disease progression in amyotrophic lateral sclerosis. Neurobiology of Disease, 114, 85–94. https://doi.org/10.1016/j.nbd.2018.02.009.
Chen, J., Zhao, B., Zhao, J., & Li, S. (2017). Potential Roles of ExosomalMicroRNAs as Diagnostic Biomarkers and Therapeutic Application in Alzheimer’s Disease. Neural Plasticity, 2017, 1–12. https://doi.org/10.1155/2017/7027380.
Reed, E. R., Latourelle, J. C., Bockholt, J. H., Bregu, J., Smock, J., Paulsen, J. S., & Myers, R. H. (2017). MicroRNAs in CSF as prodromal biomarkers for Huntington disease in the PREDICT-HD study. Neurology, 90(4), e264–e272. https://doi.org/10.1212/wnl.0000000000004844.
Weber, J. A., Baxter, D. H., Zhang, S., Huang, D. Y., How Huang, K., Jen Lee, M., Galas, D. J., & Wang, K. (2010). The MicroRNA Spectrum in 12 Body Fluids. Clinical Chemistry, 56(11), 1733–1741. https://doi.org/10.1373/clinchem.2010.147405.
Arron, T., & Wilson, C. (2016). Exosomal MiRNAs as cancer biomarkers and therapeutic targets. Journal of Extracellular Vesicles, 5, 31292. https://doi.org/10.3402/jev.v5.31292.
Lu, X., Kim-Han, J. S., & Harmon, S., et al. (2014). The Parkinsonian mimetic, 6-OHDA, impairs axonal transport in dopaminergic axons. Molecular Neurodegeneration, 9, 17. https://doi.org/10.1186/1750-1326-9-17.
Ba, Q., et al. (2015). Schisandrin B shows neuroprotective effect in 6-OHDA-Induced Parkinson’s Disease via Inhibiting the Negative Modulation of MiR-34a on Nrf2 Pathway. Biomedicine & Pharmacotherapy, 75, 165–172. https://doi.org/10.1016/j.biopha.2015.07.034.
Mao, S., et al. (2015). Secreted MiR-34a in astrocytic shedding vesicles enhanced the vulnerability of dopaminergic neurons to neurotoxins by targeting Bcl-2. Protein & Cell, 6, 529–540. https://doi.org/10.1007/s13238-015-0168-y.
Hall, J., Prabhakar, S., Balaj, L., Lai, C. P., Cerione, R. A., & Breakefield, X. O. (2016). Delivery of therapeutic proteins via extracellular vesicles: review and potential treatments for Parkinson’s disease, glioma, and schwannoma. Cellular and Molecular Neurobiology, 36(3), 417–427. https://doi.org/10.1007/s10571-015-0309-0.
Haney, M. J., Zhao, Y., Jin, Y. S., & Batrakova, E. V. (2020). Extracellular vesicles as drug carriers for enzyme replacement therapy to treat CLN2 batten disease: optimization of drug administration routes. Cells, 9(5), 1273.
Malhotra, S., Dumoga, S., Sirohi, P., & Singh, N. (2019). Red blood cells-derived vesicles for delivery of lipophilic drug camptothecin. ACS Applied Materials & Interfaces, 11(25), 22141–22151.
Wu, W.-C., Tian, J., Xiao, D., Guo, Y.-X., Xiao, Y., Wu, X.-Y., Casella, G., Rasouli, J., Yan, Y.-P., Rostami, A., Wang, L.-B., Zhang, Y., & Li, X. (2022). Engineered extracellular vesicles encapsulated Bryostatin-1 as therapy for neuroinflammation. Nanoscale, 14(6), 2393–2410. https://doi.org/10.1039/d1nr05517h.
Nakano, M. & Fujimiya, M. (2021). Potential effects of mesenchymal stem cell derived extracellular vesicles and exosomalmiRNAs in neurological disorders. Neural Regeneration Research, 16(12), 2359.
Cai, J., Wu, J., Wang, J., Li, Y., Hu, X., Luo, S., & Xiang, D. (2020). Extracellular vesicles derived from different sources of mesenchymal stem cells: therapeutic effects and translational potential. Cell & Bioscience, 10(1), 69. https://doi.org/10.1186/s13578-020-00427-x.
Liu, W., Ma, Z., Li, J., & Kang, X. (2021). Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Research & Therapy, 12(1), 102. https://doi.org/10.1186/s13287-021-02153-8.
Stevanato, L., Thanabalasundaram, L., Vysokov, N. & Sinden, J. D. (2016). Investigation of Content, Stoichiometry and Transfer of miRNA from Human Neural Stem Cell Line Derived Exosomes. PLOS ONE, 11(1), e0146353.
Iraci, N., Gaude, E., Leonardi, T., Costa, A. S. H., Cossetti, C., Peruzzotti-Jametti, L., Bernstock, J. D., Saini, H. K., Gelati, M., Vescovi, A. L., Bastos, C., Faria, N., Occhipinti, L. G., Enright, A. J., Frezza, C., & Pluchino, S. (2017). Extracellular vesicles are independent metabolic units with asparaginase activity. Nature Chemical Biology, 13(9), 951–955. https://doi.org/10.1038/nchembio.2422.
Apodaca, L. A., Baddour, A. A. D., Garcia, C., Alikhani, L., Giedzinski, E., Ru, N., Agrawal, A., Acharya, M. M., & Baulch, J. E. (2021). Human neural stem cell-derived extracellular vesicles mitigate hallmarks of Alzheimer’s disease. Alzheimer’s Research & Therapy, 13(1), 57. https://doi.org/10.1186/s13195-021-00791-x.
Wang, C., Borger, V., & Sardari, M., et al. (2020). Mesenchymal stromal cell-derived small extracellular vesicles induce ischemic neuroprotection by modulating leukocytes and specifically neutrophils. Stroke, 51, 1825–1834. https://doi.org/10.1161/STROKEAHA.119.028012.
Costa, L. A., Eiro, N., & Fraile, M., et al. (2021). Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cellular and Molecular Life Sciences, 78, 447–467. https://doi.org/10.1007/s00018-020-03600-0.
de Almeida Fuzeta, M., Bernardes, N., & Oliveira, F. D., et al. (2020). Scalable production of human mesenchymal stromal cell-derived extracellular vesicles under serum-/xeno-free conditions in a microcarrier-based bioreactor culture system. Frontiers in Cell Developmental Biology, 8, 553444. https://doi.org/10.3389/fcell.2020.553444.b500df7c63514a22843cbaa239e4e991.
Nalamolu, K. R., Venkatesh, I., & Mohandass, A., et al. (2019). Exosomes treatment mitigates ischemic brain damage but does not improve post-stroke neurological outcome. Cellular Physiology and Biochemistry, 52, 12801291. https://doi.org/10.33594/000000090.5c9033bb723d440d8d5b69c822e60a2a.
Hiroaki, K., et al. Advances of engineered extracellular vesicles-based therapeutics strategy. Science and Technology of Advanced Materials, 23(1), 655–681, www.ncbi.nlm.nih.gov/pmc/articles/PMC9586594/, https://doi.org/10.1080/14686996.2022.2133342.
Kanada, M., Bachmann, M. H., Hardy, J. W., Frimannson, D. O., Bronsart, L., Wang, A., Sylvester, M. D., Schmidt, T. L., Kaspar, R. L., Butte, M. J., Matin, A. C., & Contag, C. H. (2015). Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proceedings of the National Academy of Sciences, 112(12), E1433–E1442. https://doi.org/10.1073/pnas.1418401112.
Millard, M., Yakavets, I., Piffoux, M., Brun, A., Gazeau, F., Guigner, J.-M., Jasniewski, J., Lassalle, H.-P., Wilhelm, C., & Bezdetnaya, L. (2018). mTHPC-loaded extracellular vesicles outperform liposomal and free mTHPC formulations by an increased stability, drug delivery efficiency and cytotoxic effect in tridimensional model of tumors. Drug Delivery, 25(1), 1790–1801. https://doi.org/10.1080/10717544.2018.1513609.
Zhang, H., Wang, Y., Bai, M., Wang, J., Zhu, K., Liu, R., Ge, S., Li, J., Ning, T., Deng, T., Fan, Q., Li, H., Sun, W., Ying, G., & Ba, Y. (2018). Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Science, 109(3), 629–641. https://doi.org/10.1111/cas.13488.
Familtseva, A., Jeremic, N., & Tyagi, S. C. (2019). Exosomes: cell-created drug delivery systems. Molecular and Cellular Biochemistry, 459(1-2), 1–6. https://doi.org/10.1007/s11010-019-03545-4.
Kim, M. S., Haney, M. J., Zhao, Y., Mahajan, V., Deygen, I., Klyachko, N. L., Inskoe, E., Piroyan, A., Sokolsky, M., Okolie, O., Hingtgen, S. D., Kabanov, A. V., & Batrakova, E. V. (2016). Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine: Nanotechnology, Biology and Medicine, 12(3), 655–664. https://doi.org/10.1016/j.nano.2015.10.012.
Gong, C., Tian, J., Wang, Z., Gao, Y., Wu, X., Ding, X., Qiang, L., Li, G., Han, Z., Yuan, Y., & Gao, S. (2019). Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. Journal of Nanobiotechnology, 17, 10. https://doi.org/10.1186/s12951-019-0526-7.
Nakase, I., & Futaki, S. (2015). Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Science Report, 5, 10112.
Joshi, B. S., et al. (2020). Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano, 14(4), 4444–4455. https://doi.org/10.1021/acsnano.9b10033.
Culbreath, K., Keefe, G., & Nes, E., et al. (2024). Association between neurodevelopmental outcomes and concomitant presence of NEC and IVH in extremely low birth weight infants. Journal of Perinatology, 44, 108–115. https://doi.org/10.1038/s41372-023-01780-8.
Chen, P. Y., Hsieh, H. Y., Huang, C. Y., Lin, C. Y., Wei, K. C. & Liu, H. L. (2015). Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. Journal of Translational Medicine, 13, 93.
Yang, J., Zhang, X., Chen, X., Wang, L., & Yang, G. (2017). Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Molecular Therapy Nucleic Acids, 7, 278–287. https://doi.org/10.1016/j.omtn.2017.04.010.
Vogel, A., Upadhya, R., & Shetty, A. K. (2018). Neural stem cell derived extracellular vesicles: Attributes and prospects for treating neurodegenerative disorders. EBioMedicine, 38, 273–282. https://doi.org/10.1016/j.ebiom.2018.11.026.
Kooijmans, S. A. A., et al. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. Journal of Controlled Release: Official Journal of the Controlled Release Society, 224, 77–85, www.ncbi.nlm.nih.gov/pubmed/26773767, https://doi.org/10.1016/j.jconrel.2016.01.009.
Wiklander, O. P. B., et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. Journal of Extracellular Vesicles, 4(1), 26316. www.ncbi.nlm.nih.gov/pmc/articles/PMC4405624/, https://doi.org/10.3402/jev.v4.26316.
Dharap, A., Bowen, K., Place, R., Li, L.-C., & Vemuganti, R. (2009). Transient focal ischemia induces extensive temporal changes in rat cerebral MicroRNAome. Journal of Cerebral Blood Flow & Metabolism, 29(4), 675–687. https://doi.org/10.1038/jcbfm.2008.157.
Yin, K.-J., Deng, Z., Hamblin, M., Xiang, Y., Huang, H., Zhang, J., Jiang, X., Wang, Y., & Chen, Y. E. (2010). Peroxisome proliferator-activated receptor δ regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. Journal of Neuroscience, 30(18), 6398–6408. https://doi.org/10.1523/JNEUROSCI.0780-10.2010.
Hébert, S. S., Horré, K., Nicolaï, L., Bergmans, B., Papadopoulou, A. S., Delacourte, A., & De Strooper, B. (2009). MicroRNA regulation of Alzheimer’s Amyloid precursor protein expression. Neurobiology of Disease, 33(3), 422–428. https://doi.org/10.1016/j.nbd.2008.11.009.
Zhao, Y., Zhao, R., Wu, J., Wang, Q., Pang, K., Shi, Q., Gao, Q., Hu, Y., Dong, X., Zhang, J., & Sun, J. (2018). Melatonin protects against Aβ ‐induced neurotoxicity in primary neurons via miR‐132/PTEN/AKT/FOXO3a pathway. BioFactors, 44(6), 609–618. https://doi.org/10.1002/biof.1411.
Wang, R., Chopra, N., Nho, K., Maloney, B., Obukhov, A. G., Nelson, P. T., Counts, S. E., & Lahiri, D. K. (2022). Human microRNA (miR-20b-5p) modulates Alzheimer’s disease pathways and neuronal function, and a specific polymorphism close to the MIR20B gene influences Alzheimer’s biomarkers. Molecular Psychiatry, 27(2), 1256–1273. https://doi.org/10.1038/s41380-021-01351-3.
Kim, W., Lee, Y., McKenna, N. D., Yi, M., Simunovic, F., Wang, Y., Kong, B., Rooney, R. J., Seo, H., Stephens, R., & Sonntag, K. C. (2014). miR-126 contributes to Parkinson disease by dysregulating IGF-1/PI3K signaling. Neurobiology of Aging, 35(7), 1712–1721. https://doi.org/10.1016/j.neurobiolaging.2014.01.021.
Wang, Q., Wang, Y., Zhou, F., Li, J., Lu, G. & Zhao, Y. (2021). MiR-20a-5p Regulates MPP+-Induced Oxidative Stress and Neuroinflammation in HT22 Cells by Targeting IRF9/NF-κB Axis. Evidence-Based Complementary and Alternative Medicine, 2021, e6621206.
Kojima, R., Bojar, D., Rizzi, G., Hamri, G. C.-E., El-Baba, M. D., Saxena, P., Ausländer, S., Tan, K. R., & Fussenegger, M. (2018). Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nature Communications, 9(1), 1–10. https://doi.org/10.1038/s41467-018-03733-8.
Katakowski, M., Buller, B., Zheng, X., Lu, Y., Rogers, T., Osobamiro, O., Shu, W., Jiang, F., & Chopp, M. (2013). Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Letters, 335(1), 201–204. https://doi.org/10.1016/j.canlet.2013.02.019.
Xin, H., Li, Y., Buller, B., Katakowski, M., Zhang, Y., Wang, X., Shang, X., Zhang, Z. G., & Chopp, M. (2012). Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells, 30(7), 1556–1564. https://doi.org/10.1002/stem.1129.
Sterzenbach, U., Putz, U., Low, L.-H., Silke, J., Tan, S.-S., & Howitt, J. (2017). Engineered exosomes as vehicles for biologically active proteins. Molecular Therapy, 25(6), 1269–1278. https://doi.org/10.1016/j.ymthe.2017.03.030.
Munoz, J. L., Bliss, S. A., Greco, S. J., Ramkissoon, S. H., Ligon, K. L. & Rameshwar, P. (2013). Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Molecular Therapy - Nucleic Acids, 2, e126.
Yim, N., Ryu, S.-W., Choi, K., Lee, K. R., Lee, S., Choi, H., Kim, J., Shaker, M. R., Sun, W., Park, J.-H., Kim, D., Heo, W. D., & Choi, C. (2016). Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nature Communications, 7(1), 12277. https://doi.org/10.1038/ncomms12277.
Xia, X., et al. (2022). Exosome: a novel neurotransmission modulator or non-canonical neurotransmitter? Ageing Research Reviews, 74, 101558. https://doi.org/10.1016/j.arr.2021.101558.
Author Contributions
The authors confirm contribution to the paper as follows: Study conception and design: Dr. Humaira Farooqi. Data collection: Shahid Afridi and Pradakshina Sharma. Contributed data or analysis tools: Shahid Afridi, Pradakshina Sharma, Furqan Choudhary, Amber Rizwan, Anam Nizam. Draft manuscript preparation: Furqan Choudhary, Amber Rizwan, Anam Nizam, Adil Parvez. All authors reviewed the results and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afridi, S., Sharma, P., Choudhary, F. et al. Extracellular Vesicles: A New Approach to Study the Brain’s Neural System and Its Diseases. Cell Biochem Biophys (2024). https://doi.org/10.1007/s12013-024-01271-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s12013-024-01271-3