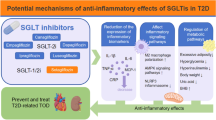
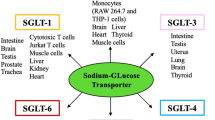
Abstract
Diabetes affects millions of people worldwide and is mainly associated with impaired insulin function. To date, various oral anti-diabetic drugs have been developed, of which, the sodium glucose transporter-2 inhibitors (SGLT2Is) are of the most recent classes that have been introduced. They differ from other classes in terms of their novel mechanism of actions and unique beneficial effects rather than just lowering glucose levels. SGLT2Is can protect body against cardiovascular events and kidney diseases even in non-diabetic individuals. SGLT2Is participate in immune cell activation, oxidative stress reduction, and inflammation mediation, thereby, moderating diabetic complications. In addition, toll like receptors (TLRs) are the intermediators of the immune system and inflammatory process, thus it’s believed to play crucial roles in diabetic complications, particularly the ones that are related to inflammatory reactions. SGLT2Is are also effective against diabetic complications via their anti-inflammatory and oxidative properties. Given the anti-inflammatory properties of TLRs and SGLT2Is, this review investigates how SGLT2Is can affect the TLR pathway, and whether this could be favorable toward diabetes.

Similar content being viewed by others
Abbreviations
- DM:
-
Diabetes
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- TLRs:
-
Toll-like receptors
- TNF:
-
Tumor necrosis factor
- NF-κB:
-
Nuclear factor kappa B
- ROS:
-
Reactive oxygen species
- ENOS:
-
Endothelial nitric oxide synthase
- PP:
-
Pancreatic polypeptide
- GLC:
-
Glucose
- BMI:
-
Body mass index
- PCOS:
-
Polycystic ovary syndrome
- HDL:
-
High density lipoprotein
- TZDs:
-
Thiazolidinediones
- DPP-4:
-
Dipeptidyl peptidase IV
- GLP-1:
-
Glucagon-like peptide-1
- SGLT-2Is:
-
Sodium-glucose cotransporter-2 inhibitors
- PAD:
-
Peripheral arterial disease
- PPR:
-
Pattern recognition receptors
- PAMPs:
-
Pathogen-associated molecular patterns
- LPS:
-
Lipopolysaccharide
- TM:
-
Transmembrane
- LRR:
-
Leucine-rich repeat
- MyD88:
-
Myeloid differentiation factor 88
- MAL:
-
MyD88-adaptor-like protein
- TRIF:
-
TIR-domain containing adaptor molecule
- TRAM:
-
TRIF-related adaptor molecule
- SARM:
-
sterile α- and armadillo-motif-containing
- IFNs:
-
Interferons
- I/R:
-
Ischemia/reperfusion
- STAT:
-
Signal transducer and activator of transcription
- iNOS:
-
Inducible nitric oxide synthase
- NO:
-
Nitric oxide
- CRP:
-
C-reactive protein
- HMGB-1:
-
High mobility group box- 1
- PPAR-γ:
-
Proliferator-activated receptor-γ
- C-C motif:
-
Chemokine
- CCL2:
-
ligand 2
- NKAP:
-
Sodium/potassium adenosine triphosphatase Na/K ATPase pump
- MI:
-
Myocardial infarction
- SOD:
-
Superoxide dismutase
- HO-1:
-
Heme oxygenase-1
- MCP-1:
-
Macrophage chemoattractant protein-1
- GPX:
-
Glutathione peroxidase
- MDA:
-
Malondialdehyde
- (NOX)-2:
-
Nicotinamide adenine dinucleotide phosphate oxidase
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- PAH:
-
Pulmonary artery hypertension
- UUO:
-
Unilateral ureteric obstruction
References
Unnikrishnan, R., Anjana, R. M., & Mohan, V. (2016). Diabetes mellitus and its complications in India. Nature Reviews Endocrinology, 12(6), 357–370.
Stedman, M., et al. (2020). Cost of hospital treatment of type 1 diabetes (T1DM) and type 2 diabetes (T2DM) compared to the non-diabetes population: a detailed economic evaluation. BMJ Open, 10(5), e033231.
Reid, L., Baxter, F., & Forbes, S. (2021). Effects of islet transplantation on microvascular and macrovascular complications in type 1 diabetes. Diabetic Medicine, 38(7), e14570.
Azhar, A., et al. (2023). Prevalence of peripheral arterial disease in diabetic foot ulcer patients and its impact in limb salvage. The International Journal of Lower Extremity Wounds, 22, 518–523.
Fowler, M. J. (2008). Microvascular and macrovascular complications of diabetes. Clinical diabetes, 26(2), 77–82.
Fowler, M. J. (2011). Microvascular and macrovascular complications of diabetes. Clinical diabetes, 29(3), 116–122.
Luc, K. et al. (2019). Oxidative stress and inflammatory markers in prediabetes and diabetes. J of Physiology and Pharmacology, 70(6), 809–824.
Zannad, F., et al. (2021). Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: insights from EMPEROR-reduced. Circulation, 143(4), 310–321.
ElSayed, N. A., et al. (2023). Summary of revisions: standards of care in diabetes-2023. Diabetes Care, 46(Suppl 1), S5–s9.
Saberzadeh-Ardestani, B., et al. (2018). Type 1 diabetes mellitus: cellular and molecular pathophysiology at a glance. Cell Journal, 20(3), 294.
Primavera, M., Giannini, C., & Chiarelli, F. (2020). Prediction and prevention of type 1 diabetes. Frontiers in Endocrinology, 11, 248.
Zorena, K., et al. (2022). Environmental factors and the risk of developing type 1 diabetes—old disease and new data. Biology, 11(4), 608.
Gamboa, D., Vázquez, C. E., & Campos, P. J. (2020). Nonlinear analysis for a type-1 diabetes model with focus on t-cells and pancreatic β-cells behavior. Mathematical and Computational Applications, 25(2), 23.
Willcox, A., et al. (2009). Analysis of islet inflammation in human type 1 diabetes. Clinical & Experimental Immunology, 155(2), 173–181.
Bottazzo, G., Florin-Christensen, A., & Doniach, D. (1974). Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. The Lancet, 304(7892), 1279–1283.
Nathan, D. (2005). for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study research group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. The New England Journal of Medicine, 353, 2643–2653.
Picardi, A., et al. (2006). Metabolic factors affecting residual beta cell function assessed by C-peptide secretion in patients with newly diagnosed type 1 diabetes. Hormone and Metabolic Research, 38(10), 668–672.
Verdu, E. F., & Danska, J. S. (2018). Common ground: shared risk factors for type 1 diabetes and celiac disease. Nature Immunology, 19(7), 685–695.
Yazdani, N. M., & Moghaddam, R. K. (2021). Blood glucose regulation in patients with type 1 diabetes by robust optimal safety critical control. Frontiers in Health Informatics, 10(1), 80.
Ghalwash, M., et al. (2022). Two-age islet-autoantibody screening for childhood type 1 diabetes: a prospective cohort study. The Lancet Diabetes & Endocrinology, 10(8), 589–596.
Patterson, C. C., et al. (2019). Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: a multicentre prospective registration study. Diabetologia, 62(3), 408–417.
Piffaretti, C., et al. (2019). Trends in childhood type 1 diabetes incidence in France, 2010–2015. Diabetes Research and Clinical Practice, 149, 200–207.
DiMeglio, L. A., Evans-Molina, C., & Oram, R. A. (2018). Type 1 diabetes. The Lancet, 391(10138), 2449–2462.
Kyrou, I., et al. (2020). Sociodemographic and lifestyle-related risk factors for identifying vulnerable groups for type 2 diabetes: a narrative review with emphasis on data from Europe. BMC endocrine disorders, 20(1), 1–13.
Yaghootkar, H., et al. (2020). Ethnic differences in adiposity and diabetes risk–insights from genetic studies. Journal of Internal Medicine, 288(3), 271–283.
Martín Giménez, V. M., et al. (2020). Differences in RAAS/vitamin D linked to genetics and socioeconomic factors could explain the higher mortality rate in African Americans with COVID-19. Therapeutic Advances in Cardiovascular Disease, 14, 1753944720977715.
Kadayifci, F. Z., et al. (2019). Early-life programming of type 2 diabetes mellitus: understanding the association between epigenetics/genetics and environmental factors. Current Genomics, 20(6), 453–463.
Seneviratne, S. N., & Rajindrajith, S. (2022). Fetal programming of obesity and type 2 diabetes. World Journal of Diabetes, 13(7), 482–497.
Tian, M., et al. (2019). Low birth weight, a risk factor for diseases in later life, is a surrogate of insulin resistance at birth. Journal of hypertension, 37(11), 2123–2134.
Farruggia, M. C., & Small, D. M. (2019). Effects of adiposity and metabolic dysfunction on cognition: a review. Physiology & Behavior, 208, 112578.
Matsuo, A. R., et al. (2020). Tri-ponderal mass index as a tool for insulin resistance prediction in overweight adolescents: a cross-sectional study. Nutrition, 74, 110744.
Lillioja, S., et al. (1988). Impaired glucose tolerance as a disorder of insulin action. New England Journal of Medicine, 318(19), 1217–1225.
Saad, M., et al. (1989). Sequential changes in serum insulin concentration during development of non-insulin-dependent diabetes. The Lancet, 333(8651), 1356–1359.
Jallut, D., et al. (1990). Impaired glucose tolerance and diabetes in obesity: A 6-year follow-up study of glucose metabolism. Metabolism, 39(10), 1068–1075.
Straczkowski, M., et al. (2003). Insulin resistance in the first-degree relatives of persons with type 2 diabetes. Medical Science Monitor, 9(5), Cr186–Cr190.
Carbone, S., et al. (2019). Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Progress in Cardiovascular Diseases, 62(4), 327–333.
Moyse, E., et al. (2019). Common pathological mechanisms and risk factors for Alzheimer’s disease and type-2 diabetes: focus on inflammation. Current Alzheimer Research, 16(11), 986–1006.
Sharma, S., & Tripathi, P. (2019). Gut microbiome and type 2 diabetes: where we are and where to go?. Journal of Nutritional Biochemistry, 63, 101–108.
Candler, T., et al. (2018). Continuing rise of type 2 diabetes incidence in children and young people in the UK. Diabetic Medicine, 35(6), 737–744.
Sun, H., et al. (2022). IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice, 183, 109119.
Bekele, H., et al. (2020). Barriers and strategies to lifestyle and dietary pattern interventions for prevention and management of type-2 diabetes in Africa, systematic review. Journal of Diabetes Research, 2020, 7948712.
Blaslov, K., et al. (2018). Treatment approach to type 2 diabetes: past, present and future. World Journal of Diabetes, 9(12), 209.
Jia, W., et al. (2019). Standards of medical care for type 2 diabetes in China 2019. Diabetes/Metabolism Research and Reviews, 35(6), e3158.
Li, M., et al. (2009). The critical role of Toll-like receptor signaling pathways in the induction and progression of autoimmune diseases. Current Molecular Medicine, 9(3), 365–374.
Arleevskaya, M. I., et al. (2020). Toll-like receptors, infections, and rheumatoid arthritis. Clinical Reviews in Allergy & Immunology, 58(2), 172–181.
Kaisho, T., & Akira, S. (2006). Toll-like receptor function and signaling. Journal of Allergy and Clinical Immunology, 117(5), 979–987.
Thompson, A. J., & Locarnini, S. A. (2007). Toll‐like receptors, RIG‐I‐like RNA helicases and the antiviral innate immune response. Immunology and Cell Biology, 85(6), 435–445.
Takeda, K., & Akira, S. (2001). Roles of Toll‐like receptors in innate immune responses. Genes to Cells, 6(9), 733–742.
Muzio, M., et al. (2000). Toll‐like receptors: a growing family of immune receptors that are differentially expressed and regulated by different leukocytes. Journal of Leukocyte Biology, 67(4), 450–456.
Akira, S., & Hemmi, H. (2003). Recognition of pathogen-associated molecular patterns by TLR family. Immunology Letters, 85(2), 85–95.
Akira, S., & Takeda, K. (2004). Toll-like receptor signalling. Nature Reviews Immunology, 4(7), 499–511.
Seki, E., & Brenner, D. A. (2008). Toll‐like receptors and adaptor molecules in liver disease: update. Hepatology, 48(1), 322–335.
Nimma, S., et al. (2021). Structural evolution of TIR-domain signalosomes. Frontiers in Immunology, 12, 784484.
Yamamoto, M., et al. (2003). Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science, 301(5633), 640–643.
Gohda, J., Matsumura, T., & Inoue, J.-I. (2004). Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not Toll/IL-1 receptor domain-containing adaptor-inducing IFN-β (TRIF)-dependent pathway in TLR signaling. The Journal of Immunology, 173(5), 2913–2917.
Kolanowski, S. T., et al. (2014). TLR4-mediated pro-inflammatory dendritic cell differentiation in humans requires the combined action of MyD88 and TRIF. Innate Immunity, 20(4), 423–430.
Yang, Y., et al. (2016). The emerging role of Toll-like receptor 4 in myocardial inflammation. Cell Death & Disease, 7(5), e2234–e2234.
Zhao, X., et al. (2018). IRF3 negatively regulates toll-like receptor-mediated NF-κB signaling by targeting TRIF for degradation in teleost fish. Frontiers in Immunology, 9, 867.
Fitzgerald, K. A., & Kagan, J. C. (2020). Toll-like receptors and the control of immunity. Cell, 180(6), 1044–1066.
Fekonja, O., Avbelj, M., & Jerala, R. (2012). Suppression of TLR signaling by targeting TIR domain-containing proteins. Current Protein and Peptide Science, 13(8), 776–788.
Cole, J. E., Georgiou, E. & Monaco, C. (2010). The expression and functions of toll-like receptors in atherosclerosis. Mediators of inflammation, 2010, ID 393946.
Li, B., Xia, Y., & Hu, B. (2020). Infection and atherosclerosis: TLR-dependent pathways. Cellular and Molecular Life Sciences, 77(14), 2751–2769.
Fresno, M., Alvarez, R., & Cuesta, N. (2011). Toll-like receptors, inflammation, metabolism and obesity. Archives of Physiology and Biochemistry, 117(3), 151–164.
Fillatreau, S., Manfroi, B., & Dörner, T. (2021). Toll-like receptor signalling in B cells during systemic lupus erythematosus. Nature Reviews Rheumatology, 17(2), 98–108.
Spirig, R., Tsui, J. & Shaw, S. (2012). The emerging role of TLR and innate immunity in cardiovascular disease. Cardiology Research and Practice, 2012, 181394.
Chedid, P., Salami, A. & El Shamieh, S. (2020). The association of rs1898830 in toll-like receptor 2 with lipids and blood pressure. Journal of Cardiovascular Development and Disease, 7(3) 24.
Ashayeri Ahmadabad, R., et al. (2021). Toll-like receptor signaling pathways: novel therapeutic targets for cerebrovascular disorders. International Journal of Molecular Science, 22(11), 6153.
Shi, H., et al. (2019). Role of Toll-like receptor mediated signaling in traumatic brain injury. Neuropharmacology, 145(Pt B), 259–267.
Leitner, G. R., et al. (2019). Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders. Expert Opinion on Therapeutic Targets, 23(10), 865–882.
Momtaz, S., et al. (2023). The Hydro-alcoholic Extract of Achillea wilhelmsii C. Koch Ameliorates Acetic Acid-induced Ulcerative Colitis through TLR-4. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 93, 127–135.
Lontchi-Yimagou, E., et al. (2013). Diabetes mellitus and inflammation. Current Diabetes Reports, 13(3), 435–444.
King, G. L. (2008). The role of inflammatory cytokines in diabetes and its complications. Journal of Periodontology, 79, 1527–1534.
Eizirik, D. L., Colli, M. L., & Ortis, F. (2009). The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nature Reviews Endocrinology, 5(4), 219–226.
Eizirik, D. L., & Colli, M. L. (2020). Revisiting the role of inflammation in the loss of pancreatic β-cells in T1DM. Nature Reviews Endocrinology, 16(11), 611–612.
Feuerer, M., et al. (2009). How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity, 31(4), 654–664.
Spranger, J., et al. (2003). Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes, 52(3), 812–817.
Pradhan, A. D., et al. (2001). C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA, 286(3), 327–334.
Tilg, H., & Moschen, A. R. (2008). Inflammatory mechanisms in the regulation of insulin resistance. Molecular Medicine, 14(3), 222–231.
Westwell‐Roper, C., et al. (2014). Toll‐like receptors and NLRP3 as central regulators of pancreatic islet inflammation in type 2 diabetes. Immunology and Cell Biology, 92(4), 314–323.
Jagannathan, M., et al. (2009). TLR cross-talk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. The Journal of Immunology, 183(11), 7461–7470.
Gupta, S., et al. (2017). Analysis of inflammatory cytokine and TLR expression levels in Type 2 Diabetes with complications. Scientific Reports, 7(1), 1–10.
Dasu, M. R., et al. (2010). Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care, 33(4), 861–868.
Mudaliar, H., et al. (2013). The role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. American Journal of Physiology-Renal Physiology, 305(2), F143–F154.
Devaraj, S., et al. (2008). Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. The Journal of Clinical Endocrinology & Metabolism, 93(2), 578–583.
Jagannathan, M., et al. (2010). Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia, 53(7), 1461–1471.
Karaali, Z. E., et al. (2019). Toll-like receptor 2 (TLR-2) gene polymorphisms in type 2 diabetes mellitus. Cell Journal, 20(4), 559.
Andrews, M., Soto, N., & Arredondo-Olguín, M. (2015). Association between ferritin and hepcidin levels and inflammatory status in patients with type 2 diabetes mellitus and obesity. Nutrition, 31(1), 51–57.
Chen, G., et al. (2017). Maternal diabetes modulates offspring cell proliferation and apoptosis during odontogenesis via the TLR 4/NF‐κB signalling pathway. Cell Proliferation, 50(3), e12324.
Devaraj, S., Tobias, P., & Jialal, I. (2011). Knockout of toll-like receptor-4 attenuates the pro-inflammatory state of diabetes. Cytokine, 55(3), 441–445.
Lin, M., et al. (2012). Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. Journal of the American Society of Nephrology, 23(1), 86–102.
Yamazaki, Y., Harada, S., & Tokuyama, S. (2018). Sodium-glucose transporter as a novel therapeutic target in disease. Europeon Journal of Pharmacology, 822, 25–31.
Gyimesi, G., et al. (2020). Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: from molecular discovery to clinical application. Pflugers Archiv, 472(9), 1177–1206.
Wright, E. M., Loo, D. D., & Hirayama, B. A. (2011). Biology of human sodium glucose transporters. Physiological Reviews, 91(2), 733–794.
Ghezzi, C., Loo, D. D. F., & Wright, E. M. (2018). Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia, 61(10), 2087–2097.
Hou, Y. C., et al. (2020). Molecular mechanisms of SGLT2 inhibitor on cardiorenal protection. Internaltional Journal of Molecular Science, 21(21) 7833.
Vallon, V., & Verma, S. (2021). Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annual Review of Physiology, 83, 503–528.
Giugliano, D., et al. (2021). Sodium-glucose transporter-2 inhibitors for prevention and treatment of cardiorenal complications of type 2 diabetes. Cardiovascular Diabetology, 20(1), 17.
Suzuki, Y., et al. (2022). Comparison of cardiovascular outcomes between SGLT2 inhibitors in diabetes mellitus. Cardiovascular Diabetology, 21(1), 67.
Dago, M., et al. (2022). Empagliflozin and dapagliflozin increase Na(+) and inward rectifier K(+) current densities in human cardiomyocytes derived from induced pluripotent stem cells (hiPSC-CMs). Cells, 11(23), 3707.
Waugh, D. T. (2019). Fluoride exposure induces inhibition of sodium-and potassium-activated adenosine triphosphatase (Na(+), K(+)-ATPase) enzyme activity: molecular mechanisms and implications for public health. International Journal of Environmental Research and Public Health, 16(8), 1427.
Fuller, W., et al. (2013). Regulation of the cardiac sodium pump. Cellular and Molecular Life Sciences, 70(8), 1357–1380.
Askari, A. (2019). The sodium pump and digitalis drugs: Dogmas and fallacies. Pharmacology Research and Perspectives, 7(4), e00505.
Kowalska, K., et al. (2021) Empagliflozin-a new chance for patients with chronic heart failure. Pharmaceuticals, 15(1), 47, https://doi.org/10.3390/ph15010047.
Refardt, J., et al. (2020). A Randomized Trial of Empagliflozin to Increase Plasma Sodium Levels in Patients with the Syndrome of Inappropriate Antidiuresis. Journal of the American Society of Nephrology, 31(3), 615–624.
Feske, S., Wulff, H., & Skolnik, E. Y. (2015). Ion channels in innate and adaptive immunity. Annual Review of Immunology, 33, 291–353.
Lees, G. J. (1991). Inhibition of sodium-potassium-ATPase: a potentially ubiquitous mechanism contributing to central nervous system neuropathology. Brain Research Reviews, 16(3), 283–300.
Li, S., & Stys, P. K. (2001). Na(+)-K(+)-ATPase inhibition and depolarization induce glutamate release via reverse Na(+)-dependent transport in spinal cord white matter. Neuroscience, 107(4), 675–683.
Stys, P. K. (2004). White matter injury mechanisms. Current Molecular Medicine, 4(2), 113–130.
Kryvenko, V., & Vadász, I. (2021). Molecular mechanisms of Na,K-ATPase dysregulation driving alveolar epithelial barrier failure in severe COVID-19. American Journal of Physiology-Lung Cellular and Molecular Physiology, 320(6), L1186–l1193.
Kabel, A. M., Estfanous, R. S., & Alrobaian, M. M. (2020). Targeting oxidative stress, proinflammatory cytokines, apoptosis and toll like receptor 4 by empagliflozin to ameliorate bleomycin-induced lung fibrosis. Respiratory Physiology and Neurobiology, 273, 103316.
Garibotto, G., et al. (2017). Toll-like receptor-4 signaling mediates inflammation and tissue injury in diabetic nephropathy. Journal of Nephrology, 30(6), 719–727.
Ashrafi Jigheh, Z., et al. (2019). Empagliflozin alleviates renal inflammation and oxidative stress in streptozotocin-induced diabetic rats partly by repressing HMGB1-TLR4 receptor axis. Iranian Journal of Basic Medical Sciences, 22(4), 384–390.
Zhang, Q. Q., et al. (2020). Empagliflozin improves chronic hypercortisolism-induced abnormal myocardial structure and cardiac function in mice. Therapeutic Advances in Chronic Disease, 11, 2040622320974833.
Gangadharan Komala, M., et al. (2014). Inhibition of kidney proximal tubular glucose reabsorption does not prevent against diabetic nephropathy in type 1 diabetic eNOS knockout mice. PLoS One, 9(11), e108994.
Al-Wakeel, D. E., El-Kashef, D. H., & Nader, M. A. (2022). Renoprotective effect of empagliflozin in cafeteria diet-induced insulin resistance in rats: Modulation of HMGB-1/TLR-4/NF-κB axis. Life Sciences, 301, 120633.
Wang, C. Y., et al. (2020). TLR9 binding to beclin 1 and mitochondrial SIRT3 by a sodium-glucose co-transporter 2 inhibitor protects the heart from doxorubicin toxicity. Biology, 9(11), 369, https://doi.org/10.3390/biology9110369.
Lee, S. G., et al. (2020). Anti-inflammatory effect for atherosclerosis progression by sodium-glucose cotransporter 2 (SGLT-2) inhibitor in a normoglycemic rabbit model. Korean Circulation Journal, 50(5), 443–457.
Qin, T., et al. (2022). Protective effects of Dapagliflozin on the vulnerability of ventricular arrhythmia in rats with pulmonary artery hypertension induced by monocrotaline. Bioengineered, 13(2), 2697–2709.
Ko, S. F., et al. (2022). Combined therapy with dapagliflozin and entresto offers an additional benefit on improving the heart function in rat after ischemia-reperfusion injury. Biomedical Journal, 46, 100546.
Refaie, M. M. M., et al. (2022). Dapagliflozin guards against cadmium-induced cardiotoxicity via modulation of IL6/STAT3 and TLR2/TNFα signaling pathways. Cardiovascular Toxicology, 22(10-11), 916–928.
Zhan, X., et al. (2022). Sodium-glucose cotransporter-2 inhibitor alleviated atrial remodeling in STZ-induced diabetic rats by targeting TLR4 pathway. Frontiers in Cardiovascular Medicine, 9, 908037.
Chen, L., Klein, T., & Leung, P. S. (2012). Effects of combining linagliptin treatment with BI-38335, a novel SGLT2 inhibitor, on pancreatic islet function and inflammation in db/db mice. Current Molecular Medicine, 12(8), 995–1004.
Nozu, T., et al. (2021). Phlorizin attenuates visceral hypersensitivity and colonic hyperpermeability in a rat model of irritable bowel syndrome. Biomedicine and Pharmacotherapy, 139, 111649.
Kabel, A. M., & Salama, S. A. (2021). Effect of taxifolin/dapagliflozin combination on colistin-induced nephrotoxicity in rats. Human and Experimental Toxicology, 40(10), 1767–1780.
Kimura, Y., et al. (2019). Canagliflozin, a sodium-glucose cotransporter 2 inhibitor, normalizes renal susceptibility to type 1 cardiorenal syndrome through reduction of renal oxidative stress in diabetic rats. Journal of Diabetes Investigation, 10(4), 933–946.
Gong, Y., et al. (2022). Effect of sotagliflozin on ventricular arrhythmias in mice with myocardial infraction. European Journal of Pharmacology, 936, 175357.
Abdollahi, E., et al. (2022). Dapagliflozin exerts anti-inflammatory effects via inhibition of LPS-induced TLR-4 overexpression and NF-κB activation in human endothelial cells and differentiated macrophages. European Journal of Pharmacology, 918, 174715.
Panchapakesan, U., et al. (2013). Effects of SGLT2 inhibition in human kidney proximal tubular cells-renoprotection in diabetic nephropathy? PLoS One, 8(2), e54442.
Dean, H. J., & Sellers, E. A. (2007). Comorbidities and microvascular complications of type 2 diabetes in children and adolescents. Pediatric Diabetes, 8(Suppl 9), 35–41.
Tomic, D., Shaw, J. E., & Magliano, D. J. (2022). The burden and risks of emerging complications of diabetes mellitus. Nature Reviews Endocrinology, 18(9), 525–539.
Kosiborod, M. N., et al. (2022). Effects of empagliflozin on symptoms, physical limitations, and quality of life in patients hospitalized for acute heart failure: results from the EMPULSE trial. Circulation, 146(4), 279–288.
Butler, J., et al. (2022). Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: the EMPEROR-preserved trial. Circulation, 145(3), 184–193.
Kosiborod, M. N., et al. (2020). Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation, 141(2), 90–99.
Sha, W., et al. (2020). The role of SGLT2 inhibitor on the treatment of diabetic retinopathy. Journal of Diabetes Research, 2020, 8867875.
Kaji, K., et al. (2018). Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. International Journal of Cancer, 142(8), 1712–1722.
Cheng, Q., et al. (2019). SP434 renal tissue proteomics changes of the early tubulointerstitial injury in diabetic rats and the protective effects of sulodexide via toll-like receptor 2/4 pathways activation. Nephrology Dialysis Transplantation, 34(Supplement_1), gfz103 SP434.
Abbas, N. A. T., El Salem, A., & Awad, M. M. (2018). Empagliflozin, SGLT(2) inhibitor, attenuates renal fibrosis in rats exposed to unilateral ureteric obstruction: potential role of klotho expression. Naunyn-Schmiedeberg’s Archives of Pharmacology, 391(12), 1347–1360.
Author information
Authors and Affiliations
Contributions
A.N., Y.H., H.S.S., A.S.K. and F.R. contributed in writing the original draft. A.N. contributed with development of figure. A.H.A. and S.M. supervised, reviewed and edited the draft. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niknejad, A., Hosseini, Y., Shamsnia, H.S. et al. Sodium Glucose Transporter-2 Inhibitors (SGLT2Is)-TLRs Axis Modulates Diabetes. Cell Biochem Biophys 81, 599–613 (2023). https://doi.org/10.1007/s12013-023-01164-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-023-01164-x