Abstract
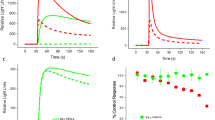
Muscarinic acetylcholine receptor subtype 3 (M3 receptor) is a G Protein-Coupled Receptor (GPCR) that mediates many important physiological functions. Currently, most M3 receptor drugs also have high affinity for other subtypes of muscarinic acetylcholine receptors (mAChRs) and produce the risk of side effects. Therefore, in order to find M3 receptor drugs with high specificity, high activity and low side effects, we established a cell model and method for efficient and sensitive screening of M3 receptor based on calcium-activated chloride channels (CaCCs), and this method is also suitable for the screening of other GPCR drugs. This screening model consists of Fischer rat thyroid follicular epithelial (FRT) cells that endogenously express M3 receptors, CaCCs, and the indicator YFP-H148Q/I152L. We verified that the model can sensitively detect changes in intracellular Ca2+ concentration using fluorescence quenching kinetics experiments, confirmed the screening function of the model by applying available M3 receptor drugs, and also evaluated the good performance of the model in high-throughput screening.





Similar content being viewed by others
References
Kruse, A. C., Kobilka, B. K., & Gautam, D., et al. (2014). Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat Rev Drug Discov, 13(7), 549–560.
Moran, S. P., Maksymetz, J., & Conn, P. J. (2019). Targeting Muscarinic Acetylcholine Receptors for the Treatment of Psychiatric and Neurological Disorders. Trends Pharmacol Sci, 40(12), 1006–1020.
Jing, M., Li, Y., & Zeng, J., et al. (2020). An optimized acetylcholine sensor for monitoring in vivo cholinergic activity. Nat Methods, 17(11), 1139–1146.
Burgio, K. L., Kraus, S. R., & Johnson, 2nd, T. M., et al. (2020). Effectiveness of Combined Behavioral and Drug Therapy for Overactive Bladder Symptoms in Men: A Randomized Clinical Trial. JAMA Intern Med, 180(3), 411–419.
Ermund, A., Meiss, L. N., & Dolan, B., et al. (2018). The mucus bundles responsible for airway cleaning are retained in cystic fibrosis and by cholinergic stimulation. Eur Respir J, 52(2), 1800457.
Kow, R. L., & Nathanson, N. M. (2012). Structural biology: Muscarinic receptors become crystal clear. Nature, 482(7386), 480–481.
Rossi, A. M., & Taylor, C. W. (2020). Reliable measurement of free Ca(2+) concentrations in the ER lumen using Mag-Fluo-4. Cell Calcium, 87, 102188.
Peters, C. J., Gilchrist, J. M., & Tien, J., et al. (2018). The Sixth Transmembrane Segment Is a Major Gating Component of the TMEM16A Calcium-Activated Chloride Channel. Neuron, 97(5), 1063–1077.e4.
Ji, Q., Guo, S., & Wang, X., et al. (2019). Recent advances in TMEM16A: Structure, function, and disease. J Cell Physiol, 234(6), 7856–7873.
Dang, S., Feng, S., & Tien, J., et al. (2017). Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature, 552(7685), 426–429.
Greenwald, E. C., Mehta, S., & Zhang, J. (2018). Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks. Chem Rev, 118(24), 11707–11794.
Rebbeck, R. T., Singh, D. P., & Janicek, K. A., et al. (2020). RyR1-targeted drug discovery pipeline integrating FRET-based high-throughput screening and human myofiber dynamic Ca(2+) assays. Sci Rep, 10(1), 1791.
Sharma, K. K., Przybilla, F., & Restle, T., et al. (2016). FRET-based assay to screen inhibitors of HIV-1 reverse transcriptase and nucleocapsid protein. Nucleic Acids Res, 44(8), e74.
Maeda, S., Qu, Q., & Robertson, M. J., et al. (2019). Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science, 364(6440), 552–557.
Bradley, S. J., Molloy, C., & Valuskova, P., et al. (2020). Biased M1-muscarinic-receptor-mutant mice inform the design of next-generation drugs. Nat Chem Biol, 16(3), 240–249.
Dana, H., Sun, Y., & Mohar, B., et al. (2019). High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat Methods, 16(7), 649–657.
de la Peña, E., & Gomis, A. (2019). Characterization of TRPC Channels in a Heterologous System Using Calcium Imaging and the Patch-Clamp Technique. Methods Mol Biol, 1987, 83–97.
Linsdell, P., Negoda, A., & Cowley, E. A., et al. (2020). Electrostatic Tuning of Anion Attraction from the Cytoplasm to the Pore of the CFTR Chloride Channel. Cell Biochem Biophys, 78(1), 15–22.
Lin, Y., Jia, Q., & Sun, W., et al. (2020). Multi targeted cell membrane chromatography: A comprehensive method for screening the anaphylactoid components from complex samples. Talanta, 209, 120539.
Wells-Cembrano, K., Sala-Jarque, J., & Del Rio, J. A. (2022). Development of a simple and versatile in vitro method for production, stimulation, and analysis of bioengineered muscle. PLoS One, 17(8), e0272610.
Yue, J. F., Qiao, G. H., & Liu, N., et al. (2016). Novel KCNQ2 channel activators discovered using fluorescence-based and automated patch-clamp-based high-throughput screening techniques. Acta Pharmacol Sin, 37(1), 105–110.
Bootman, M. D., Allman, S., & Rietdorf, K., et al. (2018). Deleterious effects of calcium indicators within cells; an inconvenient truth. Cell Calcium, 73, 82–87.
Wu, D., Gordon, C. K. L., & Shin, J. H., et al. (2022). Directed Evolution of Aptamer Discovery Technologies. Acc Chem Res, 55(5), 685–695.
Lin, C. W., & Lerner, R. A. (2021). Antibody Libraries as Tools to Discover Functional Antibodies and Receptor Pleiotropism. Int J Mol Sci, 22(8), 4123.
Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (81601234), the Science and Technology Project of Traditional Chinese Medicine of Jilin Province (2021092), the Starting fund for doctoral research of Jilin Medical University (JYBS2021013lk), the Project of Jilin Provincial Department of Education (JJKH20220462KJ, JJKH20220473SK), the Project of Science and Technology Department of Jilin Province (20220101338JC), the “Chenyu” Research and Innovation Project of Beihua University School of Medical Technology (2021006), the Project of Beihua University Graduate Innovation Program (2022021).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Ju, X., Hong, Q. et al. Establishment of a CaCC-based Cell Model and Method for High-throughput Screening of M3 Receptor Drugs. Cell Biochem Biophys 81, 49–58 (2023). https://doi.org/10.1007/s12013-022-01119-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-022-01119-8