Abstract
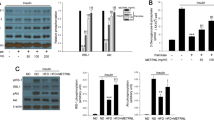
Chronic low back pain (CLBP) is a common symptom of lumbar degenerative disease. Degeneration of the lumbar paravertebral muscles causes a loss of muscle mass and strength, which is a vital factor causing CLBP and often accompanied by lipid infiltration. Tandem mass spectrometry (TMT) was used to identify differentially expressed proteins in lipid-infiltrated and normal muscles. The results show that fatty acid binding protein 4 (FABP4) participated in the peroxisome proliferator-activated receptor-γ (PPAR γ) signaling pathway as an up-regulated protein, which is related to lipogenesis in diverse cells. In addition, chronic inflammation is believed to be involved in lumbar muscle degeneration and lipogenesis, with interleukin-4 (IL-4) considered as the predominant contributor. In present study, we investigate the effect of FABP4 on lipogenesis in human skeletal muscle cells (HSMCs) stimulated by Interleukin-4 (IL-4) and explore the mechanistic basis. We found expression level of FABP4 in lipid-infiltrated muscles was significantly higher than that in normal muscles. Lipogenesis in HSMCs could be increased by IL-4 treatment, as well as by FABP4 overexpression. FABP4 inhibition suppressed IL-4-mediated lipogenesis in HSMCs, whereas the PPAR γ inhibitor alleviated lipogenesis in both IL-4-treated and FABP4-overexpressed HSMCs. Collectively, the results indicate that FABP4 induces lipogenesis in HSMCs stimulated with IL-4 via activating the PPAR γ signaling pathway. Our study offers a detailed perspective on the pathogenesis of muscle lipid infiltration and provides a potential target for the clinical treatment strategy of muscle lipid infiltration and CLBP.






Similar content being viewed by others
References
Faur, C., Patrascu, J. M., Haragus, H., & Anglitoiu, B. (2019). Correlation between multifidus lipidty atrophy and lumbar disc degeneration in low back pain. BMC Musculoskeletal Disorders, 20(1), 414 https://doi.org/10.1186/s12891-019-2786-7.
Hamrick, M. W., McGee-Lawrence, M. E., & Frechette, D. M. (2016). Lipidty infiltration of skeletal muscle: mechanisms and comparisons with bone marrow adiposity. Frontiers in Endocrinology, 7, 69 https://doi.org/10.3389/fendo.2016.00069.
Lee, S. H., Park, S. W., Kim, Y. B., Nam, T. K., & Lee, Y. S. (2017). The lipidty degeneration of lumbar paraspinal muscles on computed tomography scan according to age and disc level. The Spine Journal: Official Journal of the North American Spine Society, 17(1), 81–87. https://doi.org/10.1016/j.spinee.2016.08.001.
Stanuszek A., Jędrzejek A., Gancarczyk-Urlik E., Kołodziej I., Pisarska-Adamczyk M., Milczarek O., et al. Preoperative paraspinal and psoas major muscle atrophy and paraspinal muscle lipidty degeneration as factors influencing the results of surgical treatment of lumbar disc disease. Archives of orthopaedic and trauma surgery. 2021. https://doi.org/10.1007/s00402-021-03754-x.
Prasarn, M. L., Kostantinos, V., Coyne, E., Wright, J., & Rechtine, G. R. (2015). Does lumbar paraspinal muscle lipidty degeneration correlate with aerobic index and Oswestry disability index? Surgical Neurology International, 6(Suppl 4), S240–S243. https://doi.org/10.4103/2152-7806.156606.
Yang, C. P., Shiau, M. Y., Lai, Y. R., Ho, K. T., Hsiao, C. W., & Chen, C. J., et al. (2018). Interleukin-4 boosts insulin-induced energy deposits by enhancing glucose uptake and lipogenesis in hepatocytes. Oxidative Medicine and Cellular Longevity, 2018, 6923187 https://doi.org/10.1155/2018/6923187.
Jun, I., Kim, B. R., Park, S. Y., Lee, H., Kim, J., & Kim, E. K., et al. (2020). Interleukin-4 stimulates lipogenesis in meibocytes by activating the STAT6/PPARgamma signaling pathway. The Ocular Surface, 18(4), 575–582. https://doi.org/10.1016/j.jtos.2020.04.015.
Black, S. M., Schott, M. E., Benson, B. A., Rutherford, M. S., Young, B. K., & Dalmasso, A. P. (2008). Interleukin-4 induces lipogenesis in porcine endothelial cells, which in turn is critical for induction of protection against complement-mediated injury. Transplantation Proceedings, 40(2), 638–640. https://doi.org/10.1016/j.transproceed.2008.02.013.
Szczepankiewicz, D., Skrzypski, M., Pruszynska-Oszmalek, E., Kolodziejski, P. A., Sassek, M., & Stefanska, B., et al. (2018). Interleukin 4 affects lipid metabolism and the expression of pro-inflammatory factors in mature rat adipocytes. Immunobiology, 223(11), 677–683. https://doi.org/10.1016/j.imbio.2018.07.014.
Al-Khalili, L., Krämer, D., Wretenberg, P., & Krook, A. (2004). Human skeletal muscle cell differentiation is associated with changes in myogenic markers and enhanced insulin-mediated MAPK and PKB phosphorylation. Acta Physiologica Scandinavica, 180(4), 395–403. https://doi.org/10.1111/j.1365-201X.2004.01259.x.
Wu, W., Sun, Y., Zhao, C., Zhao, C., Chen, X., & Wang, G., et al. (2016). Lipogenesis in myoblasts and its regulation of CTRP6 by AdipoR1/Erk/PPARgamma signaling pathway. Acta Biochim Biophys Sin (Shanghai), 48(6), 509–519. https://doi.org/10.1093/abbs/gmw032.
Jun, I., Park, H. S., Piao, H., Han, J. W., An, M. J., & Yun, B. G., et al. (2017). ANO9/TMEM16J promotes tumourigenesis via EGFR and is a novel therapeutic target for pancreatic cancer. British Journal of Cancer, 117(12), 1798–1809. https://doi.org/10.1038/bjc.2017.355.
Jun, I., Lee, J. S., Lee, J. H., Lee, C. S., Choi, S. I., & Gee, H. Y., et al. (2017). Adult-onset vitelliform macular dystrophy caused by BEST1 p.Ile38Ser mutation is a mild form of best vitelliform macular dystrophy. Scientific Reports, 7(1), 9146 https://doi.org/10.1038/s41598-017-09629-9.
Liu J., Tang T., Wang G. D., Liu B. (2019). LncRNA-H19 promotes hepatic lipogenesis by directly regulating miR-130a/PPARγ axis in non-alcoholic lipidty liver disease. Bioscience Reports. 39(7). https://doi.org/10.1042/bsr20181722.
Shinohara, S., & Fujimori, K. (2020). Promotion of lipogenesis by PPARγ-activated FXR expression in adipocytes. Biochemical and Biophysical Research Communications, 527(1), 49–55. https://doi.org/10.1016/j.bbrc.2020.04.075.
Li, Z., Xu, G., Qin, Y., Zhang, C., Tang, H., & Yin, Y., et al. (2014). Ghrelin promotes hepatic lipogenesis by activation of mTOR-PPARγ signaling pathway. Proceedings of the National Academy of Sciences, 111(36), 13163–13168. https://doi.org/10.1073/pnas.1411571111.
Moratal, C., Raffort, J., Arrighi, N., Rekima, S., Schaub, S., & Dechesne, C. A., et al. (2018). IL-1β- and IL-4-polarized macrophages have opposite effects on lipogenesis of intramuscular fibro-adipogenic progenitors in humans. Scientific Reports, 8(1), 17005 https://doi.org/10.1038/s41598-018-35429-w.
Sztalryd, C., & Brasaemle, D. L. (2017). The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochimica et Biophysica Acta Molecular and Cell Biology of Lipids, 1862(10), 1221–1232. https://doi.org/10.1016/j.bbalip.2017.07.009.
Maeda, N., Funahashi, T., Matsuzawa, Y., & Shimomura, I. (2020). Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis., 292, 1–9. https://doi.org/10.1016/j.atherosclerosis.2019.10.021.
Sun, F., Du, J., Li, H., Hao, S., Zhao, G., & Lu, F. (2020). FABP4 inhibitor BMS309403 protects against hypoxia-induced H9c2 cardiomyocyte apoptosis through attenuating endoplasmic reticulum stress. Journal of Cellular and Molecular Medicine, 24(19), 11188–11197. https://doi.org/10.1111/jcmm.15666.
Seargent, J. M., Yates, E. A., & Gill, J. H. (2004). GW9662, a potent antagonist of PPARgamma, inhibits growth of breast tumour cells and promotes the anticancer effects of the PPARgamma agonist rosiglitazone, independently of PPARgamma activation. British Journal of Pharmacology, 143(8), 933–937. https://doi.org/10.1038/sj.bjp.0705973.
Battié, M. C., Joshi, A. B., & Gibbons, L. E. (2019). Degenerative disc disease: what is in a name? Spine, 44(21), 1523–1529. https://doi.org/10.1097/brs.0000000000003103.
Crawford, R. J., Volken, T., Valentin, S., Melloh, M., & Elliott, J. M. (2016). Rate of lumbar paravertebral muscle lipid infiltration versus spinal degeneration in asymptomatic populations: an age-aggregated cross-sectional simulation study. Scoliosis and Spinal Disorders, 11, 21 https://doi.org/10.1186/s13013-016-0080-0.
Kim, W. J., Shin, H. M., Lee, J. S., Song, D. G., Lee, J. W., & Chang, S. H., et al. (2021). Sarcopenia and back muscle degeneration as risk factors for degenerative adult spinal deformity with sagittal imbalance and degenerative spinal disease: a comparative study. World Neurosurgery, 148, e547–e555. https://doi.org/10.1016/j.wneu.2021.01.053.
Ohtori, S., Orita, S., Yamauchi, K., Eguchi, Y., Aoki, Y., & Nakamura, J., et al. (2016). Classification of chronic back muscle degeneration after spinal surgery and its relationship with low back pain. Asian Spine Journal, 10(3), 516–521. https://doi.org/10.4184/asj.2016.10.3.516.
Ho, I. C., & Miaw, S. C. (2016). Regulation of IL-4 expression in immunity and diseases. Advances in Experimental Medicine and Biology, 941, 31–77. https://doi.org/10.1007/978-94-024-0921-5_3.
Le Floc’h, A., Allinne, J., Nagashima, K., Scott, G., Birchard, D., & Asrat, S., et al. (2020). Dual blockade of IL-4 and IL-13 with dupilumab, an IL-4Rα antibody, is required to broadly inhibit type 2 inflammation. Allergy, 75(5), 1188–1204. https://doi.org/10.1111/all.14151.
Horsley, V., Jansen, K. M., Mills, S. T., & Pavlath, G. K. (2003). IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell, 113(4), 483–494. https://doi.org/10.1016/s0092-8674(03)00319-2.
Possidonio, A. C., Senna, M. L., Portilho, D. M., Pontes Soares, C., da Silva Sampaio, L., & Einicker-Lamas, M., et al. (2011). α-Cyclodextrin enhances myoblast fusion and muscle differentiation by the release of IL-4. Cytokine, 55(2), 280–287. https://doi.org/10.1016/j.cyto.2011.04.018.
Bao, K., & Reinhardt, R. L. (2015). The differential expression of IL-4 and IL-13 and its impact on type-2 immunity. Cytokine., 75(1), 25–37. https://doi.org/10.1016/j.cyto.2015.05.008.
Walenna, N. F., Kurihara, Y., Chou, B., Ishii, K., Soejima, T., & Itoh, R., et al. (2018). Chlamydia pneumoniae exploits adipocyte lipid chaperone FABP4 to facilitate lipid mobilization and intracellular growth in murine adipocytes. Biochemical and Biophysical Research Communications, 495(1), 353–359. https://doi.org/10.1016/j.bbrc.2017.11.005.
Qiao, Y., Liu, L., Yin, L., Xu, L., Tang, Z., & Qi, Y., et al. (2019). FABP4 contributes to renal interstitial fibrosis via mediating inflammation and lipid metabolism. Cell Death and Disease, 10(6), 382 https://doi.org/10.1038/s41419-019-1610-5.
Liu, Z. W., Fan, H. L., Liu, X. F., Ding, X. B., Wang, T., & Sui, G. N., et al. (2015). Overexpression of the A-FABP gene facilitates intermuscular lipid deposition in transgenic mice. Genetics and Molecular Research, 14(1), 2742–2749. https://doi.org/10.4238/2015.March.31.4.
Wang, Y., Liu, W., Hang, C., Du, Y., Chen, Y., & Xing, J., et al. (2019). Association of A-FABP gene polymorphism and mRNA expression with intramuscular lipid content (IMF) in Baicheng-You chicken. Journal of Animal Physiology Animal Nutrition, 103(5), 1447–1452. https://doi.org/10.1111/jpn.13150.
Zhang, L., Zhao, Y., Ning, Y., Wang, H., & Zan, L. (2017). Ectopical expression of FABP4 gene can induce bovine muscle-derived stem cells lipogenesis. Biochemical and Biophysical Research Communications, 482(2), 352–358. https://doi.org/10.1016/j.bbrc.2016.11.067.
Kim, S. W., Xie, Y., Nguyen, P. Q., Bui, V. T., Huynh, K., & Kang, J. S., et al. (2018). PPARγ regulates meibocyte differentiation and lipid synthesis of cultured human meibomian gland epithelial cells (hMGEC). The Ocular Surface, 16(4), 463–469. https://doi.org/10.1016/j.jtos.2018.07.004.
Jester, J. V., Potma, E., & Brown, D. J. (2016). PPARγ regulates mouse meibocyte differentiation and lipid synthesis. The Ocular Surface, 14(4), 484–494. https://doi.org/10.1016/j.jtos.2016.08.001.
Lamas Bervejillo, M., Bonanata, J., Franchini, G. R., Richeri, A., Marqués, J. M., & Freeman, B. A., et al. (2020). A FABP4-PPARγ signaling axis regulates human monocyte responses to electrophilic lipidty acid nitroalkenes. Redox Biology, 29, 101376 https://doi.org/10.1016/j.redox.2019.101376.
Yang, X. L., Mi, J. H., & Dong, Q. (2021). FABP4 alleviates endoplasmic reticulum stress-mediated ischemia-reperfusion injury in PC12 cells via regulation of PPARγ. Experimental and Therapeutic Medicine, 21(3), 181 https://doi.org/10.3892/etm.2021.9612.
Ayers, S. D., Nedrow, K. L., Gillilan, R. E., & Noy, N. (2007). Continuous nucleocytoplasmic shuttling underlies transcriptional activation of PPARgamma by FABP4. Biochemistry, 46(23), 6744–6752. https://doi.org/10.1021/bi700047a.
Boss, M., Kemmerer, M., Brüne, B., & Namgaladze, D. (2015). FABP4 inhibition suppresses PPARγ activity and VLDL-induced foam cell formation in IL-4-polarized human macrophages. Atherosclerosis., 240(2), 424–430. https://doi.org/10.1016/j.atherosclerosis.2015.03.042.
Acknowledgements
The authors would like to acknowledge the assistance of Dr. Chuan-dong Lang in the preparation of this article.
Author contributions
Conceived and designed the experiments: X.-W.W., Y.-J.S.; Performed the experiments: X.-W.W., Y.-J.S.; Statistical analysis: X.-W.W., X.C.; Wrote the paper: X.-W.W., Y.-J.S., W.-Z.Z. All authors read and approved the final manuscript.
Funding
This research was supported by the Fundamental Research Funds for the Central Universities (WK9110000189).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interest.
Ethics Approval and Consent to Participate
Our study was approved by the Ethics Committee of the Provincial Hospital Affiliated to Anhui Medical University and all experiments conformed to the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, Xw., Sun, Yj., Chen, X. et al. Interleukin-4-induced FABP4 promotes lipogenesis in human skeletal muscle cells by activating the PPAR γ signaling pathway. Cell Biochem Biophys 80, 355–366 (2022). https://doi.org/10.1007/s12013-022-01063-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-022-01063-7