Abstract
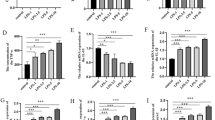
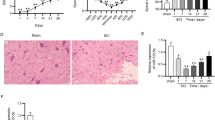
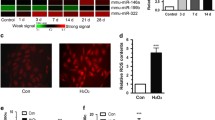
Inflammation and oxidative stress feature prominently in the secondary spinal cord injury (SCI). The present work is targeted at deciphering miR-145-5p’s role and underlying mechanism in SCI. We randomly divided Sprague-Dawley rats into SCI group and control group. Microglial BV2 cells were separated into control group and lipopolysaccharide (LPS) treatment group. Enzyme-linked immunosorbent assay was carried out for determining the concentrations of interleukin-6, interleukin-1β, and tumor necrosis factor-α (TNF-α). The expressions of malondialdehyde, glutathione peroxidase, superoxide dismutase, and reactive oxygen species were also detected. TNF-α, miR-145-5p, and Nurr1 expressions were examined by western blot and quantitative real-time polymerase chain reaction. Western blotting and dual-luciferase reporter gene assay were conducted to examine the regulating impact that miR-145-5p had on Nurr1 and TNF-α. MiR-145-5p was remarkably upregulated in the SCI rat model’s spinal cord tissues and BV2 cells treated with LPS, and Nurr1 expression was dramatically lowered. Furthermore, miR-145-5p inhibition markedly repressed inflammatory and oxidative stress responses. Moreover, it was proved that Nurr1 was a direct miR-145-5p target. The inhibition of miR-145-5p helped promote Nurr1 expression to block TNF-α signaling. MiR-145-5p inhibition mitigates inflammation and oxidative stress via targeting Nurr1 to regulate TNF-α signaling, which ameliorates SCI.




Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- SCI:
-
Spinal cord injury
- LPS:
-
Lipopolysaccharide
- ELISA:
-
Enzyme-linked immunosorbent assay
- IL-1β:
-
Interleukin-1β
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interleukin-6
- ROS:
-
Reactive oxygen species
- MDA:
-
Malondialdehyde
- GSH-Px:
-
Glutathione peroxidase
- SOD:
-
Superoxide dismutase
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- MGs:
-
Microglias
- NF-κB:
-
Nuclear factor-kappa B
- BBB:
-
Basso–Bettie–Bresnahan
- 3′-UTR:
-
3′-untranslated region
- GAPDH:
-
Gyceraldehyde 3-phosphate dehydrogenase
- miRNA:
-
MicroRNA
References
Rogers, W. K., & Todd, M. (2016). Acute spinal cord injury. Best Practice & Research: Clinical Anaesthesiology, 30(1), 27–39.
Jazayeri, S. B., Beygi, S., Shokraneh, F., Hagen, E. M., & Rahimi-Movaghar, V. (2015). Incidence of traumatic spinal cord injury worldwide: A systematic review. European Spine Journal, 24(5), 905–918.
Liu, Y., Li, Q., Zhang, B., Ban, D.-X., & Feng, S.-Q. (2017). Multifunctional biomimetic spinal cord: New approach to repair spinal cord injuries. World Journal of Experimental Medicine, 7(3), 78–83.
Orr, M. B., & Gensel, J. C. (2018). Spinal cord injury scarring and inflammation: Therapies targeting glial and inflammatory responses. Neurotherapeutics, 15(3), 541–553.
David, S., Greenhalgh, A. D., & Kroner, A. (2015). Macrophage and microglial plasticity in the injured spinal cord. Neuroscience, 307, 311–318.
Zhou, H.-J., Wang, L.-Q., Xu, Q.-S., Fan, Z.-X., Zhu, Y., Jiang, H., Zheng, X.-J., Ma, Y.-H., & Zhan, R.-Y. (2016). Downregulation of miR-199b promotes the acute spinal cord injury through IKKβ-NF-κB signaling pathway activating microglial cells. Experimental Cell Research, 349(1), 60–67.
Ning, B., Gao, L., Liu, R.-H., Liu, Y., Zhang, N.-S. & Chen, Z.-Y. (2014). microRNAs in spinal cord injury: Potential roles and therapeutic implications. International Journal of Biological Sciences, 10(9), 997–1006.
Shi, Z., Zhou, H., Lu, L., Li, X., Fu, Z., Liu, J., Kang, Y., Wei, Z., Pan, B., Liu, L., Kong, X., & Feng, S. (2017). The roles of microRNAs in spinal cord injury. The International Journal of Neuroscience, 127(12), 1104–1115.
Hachisuka, S., Kamei, N., Ujigo, S., Miyaki, S., Yasunaga, Y., & Ochi, M. (2014). Circulating microRNAs as biomarkers for evaluating the severity of acute spinal cord injury. Spinal Cord, 52(8), 596–600.
Fu, X., Shen, Y., Wang, W., & Li, X. (2018). MiR-30a-5p ameliorates spinal cord injury-induced inflammatory responses and oxidative stress by targeting Neurod 1 through MAPK/ERK signalling. Clinical and Experimental Pharmacology & Physiology, 45(1), 68–74.
He, J., Zhao, J., Peng, X., Shi, X., Zong, S., & Zeng, G. (2017). Molecular mechanism of MiR-136-5p targeting NF-κB/A20 in the IL-17-mediated inflammatory response after spinal cord injury. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 44(3), 1224–1241.
Deng, G., Gao, Y., Cen, Z., He, J., Cao, B., Zeng, G., & Zong, S. (2018). miR-136-5p regulates the inflammatory response by targeting the IKKβ/NF-κB/A20 pathway after spinal cord injury. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 50(2), 512–524.
Chen, J., Chen, T., Zhu, Y., Li, Y., Zhang, Y., Wang, Y., Li, X., Xie, X., Wang, J., Huang, M., Sun, X., & Ke, Y. (2019). circPTN sponges miR-145-5p/miR-330-5p to promote proliferation and stemness in glioma. Journal of Experimental & Clinical Cancer Research, 38(1), 398.
Cao, H., Pan, G., Tang, S., Zhong, N., Liu, H., Zhou, H., Peng, Q., & Zou, Y. (2020). miR-145-5p regulates the proliferation, migration and invasion in cervical carcinoma by targeting KLF5. OncoTargets and Therapy, 13, 2369–2376.
Zhang, H., Jiang, M., Liu, Q., Han, Z., Zhao, Y., & Ji, S. (2018). miR-145-5p inhibits the proliferation and migration of bladder cancer cells by targeting TAGLN2. Oncology Letters, 16(5), 6355–6360.
Feng, J., Zhou, Q., Yi, H., Ma, S., Li, D., Xu, Y., Wang, J., & Yin, S. (2019). A novel lncRNA n384546 promotes thyroid papillary cancer progression and metastasis by acting as a competing endogenous RNA of miR-145-5p to regulate AKT3. Cell Death & Disease, 10(6), 433.
Qi, X., Shao, M., Sun, H., Shen, Y., Meng, D., & Huo, W. (2017). Long non-coding RNA SNHG14 promotes microglia activation by regulating miR-145-5p/PLA2G4A in cerebral infarction. Neuroscience, 348, 98–106.
Xie, X., Peng, L., Zhu, J., Zhou, Y., Li, L., Chen, Y., Yu, S., & Zhao, Y. (2017). miR-145-5p/Nurr1/TNF-α signaling-induced microglia activation regulates neuron injury of acute cerebral ischemic/reperfusion in rats. Frontiers in Molecular Neuroscience, 10, 383.
Ma, S., Zhang, C., Zhang, Z., Dai, Y., Gu, R., & Jiang, R. (2019). Geniposide protects PC12 cells from lipopolysaccharide-evoked inflammatory injury via up-regulation of miR-145-5p. Artificial Cells, Nanomedicine, and Biotechnology, 47(1), 2875–2881.
Dong, J., Li, S., Mo, J.-L., Cai, H.-B., & Le, W.-D. (2016). Nurr1-based therapies for Parkinson’s disease. CNS Neuroscience & Therapeutics, 22(5), 351–359.
Moon, M., Jung, E. S., Jeon, S. G., Cha, M.-Y., Jang, Y., Kim, W., Lopes, C., Mook-Jung, I., & Kim, K.-S. (2019). Nurr1 (NR4A2) regulates Alzheimer’s disease-related pathogenesis and cognitive function in the 5XFAD mouse model. Aging Cell, 18(1), e12866.
Saijo, K., Winner, B., Carson, C. T., Collier, J. G., Boyer, L., Rosenfeld, M. G., Gage, F. H., & Glass, C. K. (2009). A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell, 137(1), 47–59.
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C., & Gage, F. H. (2010). Mechanisms underlying inflammation in neurodegeneration. Cell, 140(6), 918–934.
Wei, X., Gao, H., Zou, J., Liu, X., Chen, D., Liao, J., Xu, Y., Ma, L., Tang, B., Zhang, Z., Cai, X., Jin, K., Xia, Y., & Wang, Q. (2016). Contra-directional coupling of Nur77 and Nurr1 in neurodegeneration: A novel mechanism for memantine-induced anti-inflammation and anti-mitochondrial impairment. Molecular Neurobiology, 53(9), 5876–5892.
Pandi, P., Jain, A., Raju, S., & Khan, W. (2017). Therapeutic approaches for the delivery of TNF-α siRNA. Therapeutic Delivery, 8(5), 343–355.
Lampropoulou, I. T., Stangou, P., Sarafidis, A., Gouliovaki, P., Giamalis, I. Tsouchnikas, T., Didangelos, T. & Papagianni, A. (2020). TNF-α pathway and T-cell immunity are activated early during the development of diabetic nephropathy in Type II diabetes mellitus. Clinical Immunology, 215, 108423
Kong, X., Zhang, Z., Fu, T., Ji, J., Yang, J., & Gu, Z. (2019). TNF-α regulates microglial activation via the NF-κB signaling pathway in systemic lupus erythematosus with depression. International Journal of Biological Macromolecules, 125, 892–900.
Li, J., Lv, H., & Che, Y. (2020). microRNA-381-3p confers protection against ischemic stroke through promoting angiogenesis and inhibiting inflammation by suppressing Cebpb and Map3k8. Cellular and Molecular Neurobiology, 40(8), 1307–1319.
Zong, S., Zeng, G., Fang, Y., Peng, J., Tao, Y., Li, K., & Zhao, J. (2014). The role of IL-17 promotes spinal cord neuroinflammation via activation of the transcription factor STAT3 after spinal cord injury in the rat. Mediators of Inflammation, 2014, 786947.
Alizadeh, A., Dyck, S. M., & Karimi-Abdolrezaee, S. (2019). Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Frontiers Neurology, 10, 282.
Kabu, S., Gao, Y., Kwon, B. K., & Labhasetwar, V. (2015). Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury. Journal of Controlled Release, 219, 141–154.
Flores-Mateo, G., Elosua, R., Rodriguez-Blanco, T., Basora-Gallisà, J., Bulló, M., Salas-Salvadó, J., Martínez-González, M. Á., Estruch, R., Corella, D., Fitó, M., Fiol, M., Arós, F., Gómez-Gracia, E., Subirana, I., Lapetra, J., Ruiz-Gutiérrez, V., Sáez, G. T. & & Covas, M. I. PREDIMED Study Investigators (2014). Oxidative stress is associated with an increased antioxidant defense in elderly subjects: a multilevel approach. PLoS One, 9(9), e105881
Ponist, S., Zloh, M., Bauerova, K. (2019) Impact of oxidative stress on inflammation in rheumatoid and adjuvant arthritis: Damage to lipids, proteins, and enzymatic antioxidant defense in plasma and different tissues. Animal Models in Medicine and Biology.
Saha, A. K., Schmidt, B. R., Wilhelmy, J., Nguyen, V., & Davis, R. W. (2018). Erythrocyte deformability as a potential biomarker for chronic fatigue syndrome. Blood, 132(Suppl_1), 4874–4874.
Khansari, N., Shakiba, Y., & Mahmoudi, M. (2009). Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Patents Inflammation & Allergy Drug Discovery, 3, 73–80.
Huang, Y. N., Yang, L. Y., Greig, N. H., Wang, Y. C., Lai, C. C., & Wang, J. Y. (2018). Neuroprotective effects of pifithrin-alpha against traumatic brain injury in the striatum through suppression of neuroinflammation, oxidative stress, autophagy, and apoptosis. Scientific Reports, 8(1), 2368.
Koc, R. K., Akdemir, H., Karakücük, E. I., Oktem, I. S., & Menkü, A. (1999). Effect of methylprednisolone, tirilazad mesylate and vitamin E on lipid peroxidation after experimental spinal cord injury. Spinal Cord, 37(1), 29–32.
Genovese, T., Esposito, E., Mazzon, E., Di Paola, R., Caminiti, R., Bramanti, P., Cappelani, A., & Cuzzocrea, S. (2009). Absence of endogenous interleukin-10 enhances secondary inflammatory process after spinal cord compression injury in mice. Journal of Neurochemistry, 108(6), 1360–1372.
Lee, J. Y., Choi, H. Y., Baik, H. H., Ju, B. G., Kim, W.-K., & Yune, T. Y. (2017). Cordycepin-enriched WIB-801C from Cordyceps militaris improves functional recovery by attenuating blood-spinal cord barrier disruption after spinal cord injury. Journal of Ethnopharmacology, 203, 90–100.
Ponomarev, E. D., Veremeyko, T., & Weiner, H. L. (2013). MicroRNAs are universal regulators of differentiation, activation, and polarization of microglia and macrophages in normal and diseased CNS. Glia, 61(1), 91–103.
Rupaimoole, R., & Slack, F. J. (2017). MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nature Reviews: Drug Discovery, 16(3), 203–222.
Yunta, M., Nieto-Díaz, M., Esteban, F. J., Caballero-López, M., Navarro-Ruíz, R., Reigada, D., Pita-Thomas, D. W., del Águila, A., Muñoz-Galdeano, T., & Maza, R. M. (2012). MicroRNA dysregulation in the spinal cord following traumatic injury. PLoS One, 7(4), e34534.
Nieto-Diaz, M., Esteban, F. J., Reigada, D., Muñoz-Galdeano, T., Yunta, M., Caballero-López, M., Navarro-Ruiz, R., Del Águila, A., & Maza, R. M. (2014). MicroRNA dysregulation in spinal cord injury: causes, consequences and therapeutics. Frontiers in Cellular Neuroscience, 8, 53.
Gao, Z., Zhao, Y., He, X., Leng, Z., Zhou, X., Song, H., Wang, R., Gao, Z., Wang, Y., Liu, J., Niu, B., Li, H., Ouyang, P., & Chang, S. E. (2020). Transplantation of sh-miR-199a-5p-modified olfactory ensheathing cells promotes the functional recovery in rats with contusive spinal cord injury. Cell Transplant, 29, 963689720916173.
Jee, M. K., Jung, J. S., Im, Y. B., Jung, S. J., & Kang, S. K. (2012). Silencing of miR20a is crucial for Ngn1-mediated neuroprotection in injured spinal cord. Human Gene Therapy, 23(5), 508–520.
Gan, C. S., Wang, C. W., & Tan, K. S. (2012). Circulatory microRNA-145 expression is increased in cerebral ischemia. Genetics and Molecular Research: GMR, 11(1), 147–152.
Jankovic, J., Chen, S., & Le, W. D. (2005). The role of Nurr1 in the development of dopaminergic neurons and Parkinson’s disease. Progress in Neurobiology, 77(1-2), 128–138.
Yan, W., Chen, Z. Y., Chen, J. Q., & Chen, H. M. (2016). BMP2 promotes the differentiation of neural stem cells into dopaminergic neurons via miR-145-mediated upregulation of Nurr1 expression. American Journal of Translational Research, 8(9), 3689–3699.
Acknowledgements
We thank Hubei Yican Health Industry Co., Ltd. for its linguistic assistance during the preparation of this manuscript.
Author contributions
Conceived and designed the experiments: L.J.; performed the experiments: L.J., Z.-C.W., L.-L.X.; statistical analysis: L.-L.X., S.-Y.Y., C.L.; wrote the paper: L.J., Z.-C.W. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
Our study was approved by the Ethics Review Board of Rizhao Central Hospital and Qilu Hospital of Shandong University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, L., Wei, ZC., Xu, LL. et al. Inhibition of miR-145-5p Reduces Spinal Cord Injury-Induced Inflammatory and Oxidative Stress Responses via Affecting Nurr1-TNF-α Signaling Axis. Cell Biochem Biophys 79, 791–799 (2021). https://doi.org/10.1007/s12013-021-00992-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-021-00992-z