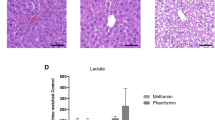
Abstract
Metformin, one of the most widely prescribed antidiabetic drugs in the world, is being repurposed as a potential drug in cancer treatment. Epidemiological studies suggest that metformin exerts anticancer effects in diabetic patients with pancreatic cancer. However, at typical antidiabetic doses the bioavailability of metformin is presumably too low to exert antitumor effects. Thus, more potent analogs of metformin are needed in order to increase its anticancer efficacy. To this end, a new class of mitochondria-targeted metformin analogs (or mito-metformins) containing a positively-charged lipophilic triphenylphosphonium group was synthesized and tested for their antitumor efficacy in pancreatic cancer cells. Results indicate that the lead compound, mito-metformin10, was nearly 1000-fold more potent than metformin in inhibiting mitochondrial complex I activity, inducing reactive oxygen species (superoxide and hydrogen peroxide) that stimulate redox signaling mechanisms, including the activation of adenosinemonophosphate kinase and inhibition of proliferation of pancreatic cancer cells. The potential use of the low-temperature electron paramagnetic resonance technique in assessing the role of mitochondrial complexes including complex I in tumor regression in response to metformin and mito-metformins in the in vivo setting is discussed.






Similar content being viewed by others
References
Bailey, C., & Day, C. (2004). Metformin: Its botanical background. Practical Diabetes International, 21, 115–117.
Thomas, I., & Gregg, B. (2017). Metformin; A review of its history and future: From lilac to longevity. Pediatric Diabetes, 18, 10–16.
Evans, J. M., Donnelly, L. A., Emslie-Smith, A. M., Alessi, D. R., & Morris, A. D. (2005). Metformin and reduced risk of cancer in diabetic patients. BMJ (Clinical Research Ed.), 330, 1304–1305.
Heckman-Stoddard, B. M., Gandini, S., Puntoni, M., Dunn, B. K., Decensi, A., & Szabo, E. (2016). Repurposing old drugs to chemoprevention: The case of metformin. Seminars in Oncology, 43, 123–133.
Kordes, S., Pollak, M. N., Zwinderman, A. H., Mathot, R. A., Weterman, M. J., Beeker, A., Punt, C. J., Richel, D. J., & Wilmink, J. W. (2015). Metformin in patients with advanced pancreatic cancer: A double-blind, randomised, placebo-controlled phase 2 trial. The Lancet Oncology, 16, 839–847.
Chandel, N. S., Avizonis, D., Reczek, C. R., Weinberg, S. E., Menz, S., Neuhaus, R., Christian, S., Haegebarth, A., Algire, C., & Pollak, M. (2016). Are metformin doses used in murine cancer models clinically relevant? Cell Metabolism, 23, 569–570.
Cheng, G., Zielonka, J., Ouari, O., Lopez, M., McAllister, D., Boyle, K., Barrios, C. S., Weber, J. J., Johnson, B. D., Hardy, M., Dwinell, M. B., & Kalyanaraman, B. (2016). Mitochondria-targeted analogues of metformin exhibit enhanced antiproliferative and radiosensitizing effects in pancreatic cancer cells. Cancer Research, 76, 3904–3915.
Graham, G. G., Punt, J., Arora, M., Day, R. O., Doogue, M. P., Duong, J. K., Furlong, T. J., Greenfield, J. R., Greenup, L. C., Kirkpatrick, C. M., Ray, J. E., Timmins, P., & Williams, K. M. (2011). Clinical pharmacokinetics of metformin. Clinical Pharmacokinetics, 50, 81–98.
Foretz, M., Guigas, B., Bertrand, L., Pollak, M., & Viollet, B. (2014). Metformin: From mechanisms of action to therapies. Cell Metabolism, 20, 953–966.
Bridges, H., Jones, A., Pollak, M., & Hirst, J. (2014). Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. The Biochemical Journal, 462, 475–487.
Liu, X., Romero, I. L., Litchfield, L. M., Lengyel, E., & Locasale, J. W. (2016). Metformin targets central carbon metabolism and reveals mitochondrial requirements in human cancers. Cell Metabolism, 24, 728–739.
Wheaton, W. W., Weinberg, S. E., Hamanaka, R. B., Soberanes, S., Sullivan, L. B., Anso, E., Glasauer, A., Dufour, E., Mutlu, G. M., Budigner, G. S., & Chandel, N. S. (2014). Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife, 3, e02242.
Birsoy, K., Possemato, R., Lorbeer, F. K., Bayraktar, E. C., Thiru, P., Yucel, B., Wang, T., Chen, W. W., Clish, C. B., & Sabatini, D. M. (2014). Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature, 508, 108–112.
Pollak, M. (2014). Overcoming drug development bottlenecks with repurposing: Repurposing biguanides to target energy metabolism for cancer treatment. Nature Medicine, 20, 591–593.
Eikawa, S., Nishida, M., Mizukami, S., Yamazaki, C., Nakayama, E., & Udono, H. (2015). Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proceedings of the National Academy of Sciences, 112, 1809–1814.
Webb, T. J., Carey, G. B., East, J. E., Sun, W., Bollino, D. R., Kimball, A. S., Brutkiewicz, R. R., & Flajnik, M. (2016). Alterations in cellular metabolism modulate CD1d-mediated NKT-cell responses. Pathogens and Disease, 74, ftw055–ftw055.
Delmastro-Greenwood, M. M., & Piganelli, J. D. (2013). Changing the energy of an immune response. American Journal of Clinical and Experimental Immunology, 2, 30–54.
Cheng, G., Zielonka, J., McAllister, D., Hardy, M., Ouari, O., Joseph, J., Dwinell, M. B., & Kalyanaraman, B. (2015). Antiproliferative effects of mitochondria-targeted cationic antioxidants and analogs: Role of mitochondrial bioenergetics and energy-sensing mechanism. Cancer Letters, 365, 96–106.
Cheng, G., Zielonka, J., Dranka, B. P., McAllister, D., Mackinnon, Jr., A. C., Joseph, J., & Kalyanaraman, B. (2012). Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Research, 72, 2634–2644.
Cheng, G., Zielonka, J., McAllister, D., Tsai, S., Dwinell, M. B., & Kalyanaraman, B. (2014). Profiling and targeting of cellular bioenergetics: Inhibition of pancreatic cancer cell proliferation. British Journal of Cancer, 111, 85–93.
Cheng, G., Zielonka, J., McAllister, D. M., Mackinnon, Jr., A. C., Joseph, J., Dwinell, M. B., & Kalyanaraman, B. (2013). Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC Cancer, 13, 285.
Dickey, J. S., Gonzalez, Y., Aryal, B., Mog, S., Nakamura, A. J., Redon, C. E., Baxa, U., Rosen, E., Cheng, G., Zielonka, J., Parekh, P., Mason, K. P., Joseph, J., Kalyanaraman, B., Bonner, W., Herman, E., Shacter, E., & Rao, V. A. (2013). Mito-tempol and dexrazoxane exhibit cardioprotective and chemotherapeutic effects through specific protein oxidation and autophagy in a syngeneic breast tumor preclinical model. PLoS ONE, 8, e70575.
Rao, V. A., Klein, S. R., Bonar, S. J., Zielonka, J., Mizuno, N., Dickey, J. S., Keller, P. W., Joseph, J., Kalyanaraman, B., & Shacter, E. (2010). The antioxidant transcription factor Nrf2 negatively regulates autophagy and growth arrest induced by the anticancer redox agent mitoquinone. The Journal of Biological Chemistry, 285, 34447–34459.
Zhao, H., Joseph, J., Fales, H. M., Sokoloski, E. A., Levine, R. L., Vasquez-Vivar, J., & Kalyanaraman, B. (2005). Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proceedings of the National Academy of Sciences of the United States of America, 102, 5727–5732.
Zielonka, J., & Kalyanaraman, B. (2010). Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth. Free Radical Biology & Medicine, 48, 983–1001.
Zielonka, J., Vasquez-Vivar, J., & Kalyanaraman, B. (2008). Detection of 2-hydroxyethidium in cellular systems: A unique marker product of superoxide and hydroethidine. Nature Protocols, 3, 8–21.
Zielonka, J., Hardy, M., & Kalyanaraman, B. (2009). HPLC study of oxidation products of hydroethidine in chemical and biological systems: Ramifications in superoxide measurements. Free Radical Biology and Medicine, 46, 329–338.
Zielonka, J., Srinivasan, S., Hardy, M., Ouari, O., Lopez, M., Vasquez-Vivar, J., Avadhani, N. G., & Kalyanaraman, B. (2008). Cytochrome c-mediated oxidation of hydroethidine and mito-hydroethidine in mitochondria: Identification of homo- and heterodimers. Free Radical Biology and Medicine, 44, 835–846.
Sikora, A., Zielonka, J., Adamus, J., Debski, D., Dybala-Defratyka, A., Michalowski, B., Joseph, J., Hartley, R. C., Murphy, M. P., & Kalyanaraman, B. (2013). Reaction between peroxynitrite and triphenylphosphonium-substituted arylboronic acid isomers: Identification of diagnostic marker products and biological implications. Chemical Research in Toxicology, 26, 856–867.
Zielonka, J., Sikora, A., Adamus, J., & Kalyanaraman, B. (2015). Detection and differentiation between peroxynitrite and hydroperoxides using mitochondria-targeted arylboronic acid. Methods in Molecular Biology, 1264, 171–181.
Zielonka, J., Zielonka, M., VerPlank, L., Cheng, G., Hardy, M., Ouari, O., Ayhan, M. M., Podsiadly, R., Sikora, A., Lambeth, J. D., & Kalyanaraman, B. (2016). Mitigation of NADPH oxidase 2 activity as a strategy to inhibit peroxynitrite formation. The Journal of Biological chemistry, 291, 7029–7044.
Hardie, D. G., & Ashford, M. L. (2014). AMPK: Regulating energy balance at the cellular and whole body levels. Physiology (Bethesda, Md.), 29, 99–107.
Mackenzie, R. M., Salt, I. P., Miller, W. H., Logan, A., Ibrahim, H. A., Degasperi, A., Dymott, J. A., Hamilton, C. A., Murphy, M. P., Delles, C., & Dominiczak, A. F. (2013). Mitochondrial reactive oxygen species enhance AMP-activated protein kinase activation in the endothelium of patients with coronary artery disease and diabetes. Clinical Science 124, 403–411.
Quintero, M., Colombo, S. L., Godfrey, A., & Moncada, S. (2006). Mitochondria as signaling organelles in the vascular endothelium. Proceedings of the National Academy of Sciences of the United States of America, 103, 5379–5384.
Boukalova, S., Stursa, J., Werner, L., Ezrova, Z., Cerny, J., Bezawork-Geleta, A., Pecinova, A., Dong, L., Drahota, Z., & Neuzil, J. (2016). Mitochondrial targeting of metformin enhances its activity against pancreatic cancer. Molecular Cancer Therapeutics, 15, 2875–2886.
Orme-Johnson, N. R., Hansen, R. E., & Beinert, H. (1974). Electron paramagnetic resonance-detectable electron acceptors in beef heart mitochondria. Reduced diphosphopyridine nucleotide ubiquinone reductase segment of the electron transfer system. The Journal of Biological Chemistry, 249, 1922–1927.
Chandran, K., Aggarwal, D., Migrino, R. Q., Joseph, J., McAllister, D., Konorev, E. A., Antholine, W. E., Zielonka, J., Srinivasan, S., Avadhani, N. G., & Kalyanaraman, B. (2009). Doxorubicin inactivates myocardial cytochrome c oxidase in rats: Cardioprotection by Mito-Q. Biophysical Journal, 96, 1388–1398.
Bennett, B., Helbling, D., Meng, H., Jarzembowski, J., Geurts, A. M., Friederich, M. W., Van Hove, J. L. K., Lawlor, M. W., & Dimmock, D. P. (2016). Potentially diagnostic electron paramagnetic resonance spectra elucidate the underlying mechanism of mitochondrial dysfunction in the deoxyguanosine kinase deficient rat model of a genetic mitochondrial DNA depletion syndrome. Free Radical Biology and Medicine, 92, 141–151.
Ghosh, A., Chandran, K., Kalivendi, S. V., Joseph, J., Antholine, W. E., Hillard, C. J., Kanthasamy, A., Kanthasamy, A., & Kalyanaraman, B. (2010). Neuroprotection by a mitochondria-targeted drug in a Parkinson’s disease model. Free Radical Biology & Medicine, 49, 1674–1684.
Sobotta, M. C., Liou, W., Stöcker, S., Talwar, D., Oehler, M., Ruppert, T., Scharf, A. N. D., & Dick, T. P. (2015). Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nature Chemical Biology, 11, 64–70.
Babot, M., Birch, A., Labarbuta, P., & Galkin, A. (2014). Characterisation of the active/de-active transition of mitochondrial complex I. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1837, 1083–1092.
Funding
This work was supported by a grant from NIH (U01 CA178960) to M.D. and B.K. A.S. was supported by a grant from Polish National Science Centre No. 2015/18/E/ST4/00235.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kalyanaraman, B., Cheng, G., Hardy, M. et al. Modified Metformin as a More Potent Anticancer Drug: Mitochondrial Inhibition, Redox Signaling, Antiproliferative Effects and Future EPR Studies. Cell Biochem Biophys 75, 311–317 (2017). https://doi.org/10.1007/s12013-017-0796-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-017-0796-3