Abstract
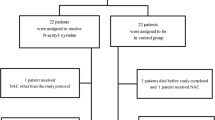
Our objective was to investigate the effect of continuous therapeutic sedation on the immune response, plasma levels of antioxidants, and tissue repair indicators in burn-induced sepsis patients. A total of 104 burn-induced sepsis patients hospitalized during March, 2008 to March, 2013 were selected for the study and randomly divided into the experimental and control groups, each with 53 cases. All of these patients received conventional treatment and the patients in the experimental group were given an additional therapy of continuous sedation. The number of T lymphocytes, plasma levels of tissue repair indicators, and antioxidants were measured before and after the treatment. Continuous midazolam treatment induced a significant increase in plasma levels of gelsolin, heat shock protein 70, nitric oxide, superoxide dismutase, and tumor necrosis factor-alpha (p < 0.05). Likewise, the relative counts of CD3+, CD4+, CD8+ T lymphocytes, T cells exhibiting HLA-DR and CD4+/CD8+ ratio were significantly increased in the patients treated with midazolam. No adverse reaction including respiratory depression, midazolam resistance, or withdrawal syndrome was observed. Continuous sedation therapy was found to enhance immune response, increase the plasma levels of antioxidants, and tissue protective/repair mediators in burn-induced sepsis patients. This therapy caused no adverse reaction or over-inhibition of the oxidative stress suggesting its effectiveness in improving the prognosis without the risk of safety.
Similar content being viewed by others
References
Nguyen, H. B., & Smith, D. (2007). Sepsis in the 21st century: Recent definitions and therapeutic advances. American Journal of Emergency Medicine, 25, 564–571.
Liu, Y., Zhou, Q., Wang, Y., Liu, Z., Dong, M., Li, X., & Hu, D. (2014). Negative pressure wound therapy decreases mortality in a murine model of burn-wound sepsis involving Pseudomonas aeruginosa infection. PLoS One, 9(2), e90494.
Yizhi, P., Jing, C., Zhiqiang, Y., Xiaolu, L., Gaoxing, L., & Jun, W. (2013). Diagnostic criteria and treatment protocol for post-burn sepsis. Critical Care, 17(1), 406.
Gentile, L. F., & Moldawer, L. L. (2012). What is the role for the inflammasome in burn injury and sepsis? Shock, 37(1), 124–125.
Zu, H., Li, Q., & Huang, P. (2014). Expression of treg subsets on intestinal T cell immunity and endotoxin translocation in porcine sepsis after severe burns. Cell Biochemistry and Biophysics, 70(3), 1699–1704.
Quoilin, C., Mouithys-Mickalad, A., Lecart, S., Fontaine-Aupart, M. P., & Hoebeke, M. (2014). Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1837(10), 1790–1800.
Ince, C. (2005). The microcirculation is the motor of sepsis. Critical Care, 9, S13–S19.
Erbas, O., & Taskiran, D. (2014). Sepsis-induced changes in behavioral stereotypy in rats; Involvement of tumor necrosis factor-alpha, oxidative stress, and dopamine turnover. Journal of Surgical Research, 186(1), 262–268.
Lange, M., Szabo, C., Traber, D. L., Horvath, E., Hamahata, A., Nakano, Y., et al. (2012). Time profile of oxidative stress and neutrophil activation in ovine acute lung injury and sepsis. Shock, 37(5), 468–472.
Dellinger, R. P., Levy, M. M., Carlet, J. M., Bion, J., Parker, M. M., Jaeschke, R., et al. (2008). Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2008. Critical Care Medicine, 36(1), 296–327.
Dawson, R., von Fintel, N., & Nairn, S. (2010). Sedation assessment using the Ramsay scale. Emergency Nurse, 18(3), 18–20.
Brunner, R., Rinner, W., Haberler, C., Kitzberger, R., Sycha, T., Herkner, H., et al. (2013). Early treatment with IgM-enriched intravenous immunoglobulin does not mitigate critical illness polyneuropathy and/or myopathy in patients with multiple organ failure and SIRS/sepsis: A prospective, randomized, placebo-controlled, double-blinded trial. Critical Care, 17(5), R213.
Klein Klouwenberg, P. M., Ong, D. S., Bonten, M. J., & Cremer, O. L. (2012). Classification of sepsis, severe sepsis and septic shock: the impact of minor variations in data capture and definition of SIRS criteria. Intensive Care Medicine, 38(5), 811–819.
Rixen, D., Siegel, J. H., & Friedman, H. P. (1996). “Sepsis/SIRS,” physiologic classification, severity stratification, relation to cytokine elaboration and outcome prediction in posttrauma critical illness. Journal of Trauma, 41(4), 581–598.
Pepin, E., Higa, A., Schuster-Klein, C., Bernard, C., Sulpice, T., Guardiola, B., et al. (2014). Deletion of apoptosis signal-regulating kinase 1 (ASK1) protects pancreatic beta-cells from stress-induced death but not from glucose homeostasis alterations under pro-inflammatory conditions. PLoS One, 9(11), e112714.
Adib-Conquy, M., & Cavaillon, J. M. (2007). Stress molecules in sepsis and systemic inflammatory response syndrome. FEBS Letters, 581, 3723–3733.
Bar-Or, D., Bar-Or, R., Rael, L. T., & Brody, E. N. (2015). Oxidative stress in severe acute illness. Redox Biology. doi:10.1016/j.redox.2015.01.006.
Preiser, J. C., Reper, P., Vlasselaer, D., Vray, B., Zhang, H., Metz, G., et al. (1996). Nitric oxide production is increased in patients after burn injury. Journal of Trauma, 40(3), 368–371.
Wallace, J. L. (2005). Nitric oxide as a regulator of inflammatory processes. Memorias do Instituto Oswaldo Cruz, 100(Suppl. I), 5–9.
Akbas, A., Inanir, A., Benli, I., Onder, Y., & Aydogan, L. (2014). Evaluation of some antioxidant enzyme activities (SOD and GPX) and their polymorphisms (MnSOD2 Ala9Val, GPX1 Pro198Leu) in fibromyalgia. European Review for Medical and Pharmacology Sciences, 18(8), 1199–1203.
Afolayan, A. J., Teng, R. J., Eis, A., Rana, U., Broniowska, K. A., Corbett, J. A., et al. (2014). Inducible HSP70 regulates superoxide dismutase-2 and mitochondrial oxidative stress in the endothelial cells from developing lungs. American Journal of Physiology-Lung Cellular and Molecular Physiology, 306(4), L351–L360.
Deb, R., Sajjanar, B., Singh, U., Kumar, S., Brahmane, M. P., Singh, R., et al. (2013). Promoter variants at AP2 box region of Hsp70.1 affect thermal stress response and milk production traits in Frieswal cross bred cattle. Gene, 532(2), 230–235.
McConnell, K. W., Fox, A. C., Clark, A. T., et al. (2011). The role of HSP70 in mediating age-dependent mortality in sepsis. Journal of Immunology, 186(6), 3718–3725.
Ding, X. Z., Fernandez-Prada, C. M., Bhattacharjee, A. K., & Hoover, D. L. (2001). Over-expression of hsp-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine, 16, 210–219.
Kuang, X., Ma, K., & Duan, T. (2002). The significance of postburn changes in plasma levels of ICAM-1, IL-10 and TNFalpha during early postburn stage in burn patients. Zhonghua Shao Shang Za Zhi, 18(5), 302–304.
Marano, M. A., Fong, Y., Moldawer, L. L., Wei, H., Calvano, S. E., Tracey, K. J., et al. (1990). Serum cachectin/tumor necrosis factor in critically ill patients with burns correlates with infection and mortality. Surgical, Gynecology and Obstetrics, 170(1), 32–38.
Arslan, E., Yavuz, M., & Dalay, C. (2000). The relationship between tumor necrosis factor (TNF)-alpha and survival following granulocyte-colony stimulating factor (G-CSF) administration in burn sepsis. Burns, 26(6), 521–524.
Al-Shudiefat, A. A., Sharma, A. K., Bagchi, A. K., Dhingra, S., & Singal, P. K. (2013). Oleic acid mitigates TNF-alpha-induced oxidative stress in rat cardiomyocytes. Molecular and Cellular Biochemistry, 372(1–2), 75–82.
Babu, D., Soenen, S. J., Raemdonck, K., Leclercq, G., De Backer, O., Motterlini, R., & Lefebvre, R. A. (2012). TNF-alpha/cycloheximide-induced oxidative stress and apoptosis in murine intestinal epithelial MODE-K cells. Current Pharmaceutical Design, 18(28), 4414–4425.
Madani, Z., Louchami, K., Sener, A., Malaisse, W. J., & Ait Yahia, D. (2012). Dietary sardine protein lowers insulin resistance, leptin and TNF-alpha and beneficially affects adipose tissue oxidative stress in rats with fructose-induced metabolic syndrome. International Journal of Molecular Medicine, 29(2), 311–318.
Kim, S. N., Son, S. C., Lee, S. M., et al. (2006). Midazolam inhibits proinflammatory mediators in the lipopolysaccharide-activated macrophage. Anesthesiology, 105, 105–110.
Anderson, S. L., Duke-Novakovski, T., & Singh, B. (2014). The immune response to anesthesia: Part 2 sedatives, opioids, and injectable anesthetic agents. Veterinary Anaesthesia and Analgesia, 41, 553–566.
Maniatis, N. A., Harokopos, V., Thanassopoulou, A., Oikonomou, N., Mersinias, V., Witke, W., et al. (2009). A critical role for gelsolin in ventilator-induced lung injury. American Journal of Respiratory Cell and Molecular Biology, 41(4), 426–432.
Vincent, J. L., Opal, S. M., Marshall, J. C., & Tracey, K. J. (2013). Sepsis definitions: time for change. Lancet, 381(9868), 7.
Entezami, K. Z., Khosravi, A., Mousavi, T., & Bahar, M. A. (2010). Immunophenotype of peripheral blood lymphocytes following thermal injury in patients. Medical Journal of The Islamic Republic of Iran, 24(2), 96–102.
Sparkes, B. G. (1997). Immunological responses to thermal injury. Burns, 23, 106–113.
Huemer, H. P., Lassnig, C., Nowotny, N., et al. (2010). Diazepam leads to enhanced severity of orthopoxvirus infection and immune suppression. Vaccine, 28, 6152–6158.
van den Berk, J. M. M., Oldenburger, R. H. J., van den Berg, A. P., Klompmaker, I. J., Mesander, G., van Son, W. J., et al. (1997). Low HLA DR expression on monocytes as a prognostic marker for bacterial sepsis after liver transplantation. Transplantation, 63, 1846–1848. 774–775.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Meng, K., Su, W. et al. The Effect of Continuous Sedation Therapy on Immunomodulation, Plasma Levels of Antioxidants, and Indicators of Tissue Repair in Post-Burn Sepsis Patients. Cell Biochem Biophys 73, 473–478 (2015). https://doi.org/10.1007/s12013-015-0681-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-015-0681-x