Abstract
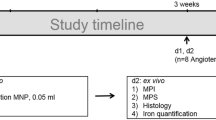
This study’s goal was to assess the diagnostic value of the USPIO-(ultra-small superparamagnetic iron oxide) enhanced magnetic resonance imaging (MRI) in detection of vulnerable atherosclerotic plaques in abdominal aorta in experimental atherosclerosis. Thirty New Zealand rabbits were randomly divided into two groups, Group A and Group B. Each group comprised 15 animals which were fed with high cholesterol diet for 8 weeks and then subjected to balloon-induced endothelial injury of the abdominal aorta. After another 8 weeks, animals in Group B received adenovirus carrying p53 gene that was injected through a catheter into the aortic segments rich in plaques. Two weeks later, all rabbits were challenged with the injection of Chinese Russell’s viper venom and histamine. Pre-contrast images and USPIO-enhanced MRI images were obtained after pharmacological triggering with injection of USPIO for 5 days. Blood specimens were taken for biochemical and serological tests at 0 and 18 weeks. Abdominal aorta was histologically studied. The levels of serum ICAM-1 and VCAM-1 were quantified by ELISA. Vulnerable plaques appeared as a local hypo-intense signal on the USPIO-enhanced MRI, especially on T2*-weighted sequences. The signal strength of plaques reached the peak at 96 h. Lipid levels were significantly (p < 0.05) higher in both Group A and B compared with the levels before the high cholesterol diet. The ICAM-1 and VCAM-1 levels were significantly (p < 0.05) higher in Group B compared with Group A. The USPIO-enhanced MRI efficiently identifies vulnerable plaques due to accumulation of USPIO within macrophages in abdominal aorta plaques.




Similar content being viewed by others
Abbreviations
- USPIO:
-
Ultra-small superparamagnetic iron oxide
- MRI:
-
Magnetic resonance imaging
- T2*WI:
-
T2*-weighted imaging
- T1WI:
-
T1-weighted imaging
- T2WI:
-
T2-weighted imaging
- PDWI:
-
Proton density weighted imaging
- ICAM-1:
-
Intercellular adhesion molecule-1
- VCAM-1:
-
Vascular cell adhesion molecule-1
- ACS:
-
Acute coronary syndrome
- SNR:
-
Signal to noise ratio
- SI:
-
Signal interesting
- SD:
-
Standard deviation
- ANOVA:
-
Analysis of variance
- FSE:
-
Fast spin echo
- TR:
-
Repetition time
- TE:
-
Echo time
- FA:
-
Flip angle
- STIR:
-
Short time inversion recovery
- FFE:
-
Fast field echo
- TC:
-
Total cholesterols
- TG:
-
Triglycerides
- LDL-C:
-
Low density lipoprotein-cholesterol
- HDL-C:
-
High density lipoprotein-cholesterol
- ELISA:
-
Enzyme-linked immunosorbent assay
- SGM:
-
Susceptibility gradient mapping
- VSOP:
-
Very small iron oxide particle
References
Fishbein, M. C. (2010). The vulnerable and unstable atherosclerotic plaque. Cardiovascular Pathology, 2010(19), 6–11.
Mizuno, Y., Jacob, R. F., & Mason, R. P. (2011). Inflammation and the development of atherosclerosis. Journal of Atherosclerosis and Thrombosis, 18, 351–358.
Nguyen, C. M., & Levy, A. J. (2010). The mechanics of atherosclerotic plaque rupture by inclusion/matrix interfacial decohesion. Journal of Biomechanics, 2010(43), 2702–2708.
Bond, A. R., & Jackson, C. L. (2011). The fat-fed apolipoprotein E knockout mouse brachiocephalic artery in the study of atherosclerotic plaque rupture. Journal of Biomedicine and Biotechnology, 2011(2011), 379069.
Guagliumi, G., Musumeci, G., Pierli, C., Fineschi, M., & Musuraca, A. C. (2010). Imaging of vulnerable plaque. Giornale Italiano Cardiologia (Rome), 11, 16s–21s.
Falk, E., Shah, P. K., & Fuster, V. (1995). Coronary plaque disruption. Circulation, 92, 657–671.
Naghavi, M., Libby, P., Falk, E., Casscells, S. W., Litovsky, S., Rumberger, J., et al. (2003). From vulnerable plaque to vulnerable patient: A call for new definitions and risk assessment strategies: Part I. Circulation, 2003(108), 1664–1672.
Fleiner, M., Kummer, M., Mirlacher, M., Sauter, G., Cathomas, G., Krapf, R., & Biedermann, B. C. (2004). Arterial neovascularization and inflammation in vulnerable patients: Early and late signs of symptomatic atherosclerosis. Circulation, 110, 2843–2850.
Staub, D., Schinkel, A. F., Coll, B., Coli, S., van der Steen, A. F., Reed, J. D., et al. (2010). Contrast-enhanced ultrasound imaging of the vasa vasorum: From early atherosclerosis to the identification of unstable plaques. JACC Cardiovascular Imaging., 3, 761–771.
Skotland, T. (2012). Molecular imaging: Challenges of bringing imaging of intracellular targets into common clinical use. Contrast Media & Molecular Imaging, 7, 1–6.
Nemoto, S. (2011). Diagnostic imaging of carotid stenosis: Ultrasound, magnetic resonance imaging, and computed tomography angiography. Nihon Geka Gakkai Zasshi, 112, 371–376. In Japanese.
Gao, T., He, X., Yu, W., Zhang, Z., & Wang, Y. (2011). Atherosclerotic plaque pathohistology and classification with high-resolution MRI. Neurological Research, 33, 325–330.
Ross, R. (1999). Atherosclerosis—an inflammatory disease. New England Journal of Medicine, 340, 115–126.
Tang, T., Howarth, S. P., Miller, S. R., Trivedi, R., Graves, M. J., King-Im, J. U., et al. (2006). Assessment of inflammatory burden contralateral to the symptomatic carotid stenosis using high-resolution ultrasmall, superparamagnetic iron oxide-enhanced MRI. Stroke, 37, 2266–2270.
Howarth, S. P., Tang, T. Y., Graves, M. J., U-King-Im, J. M., Li, Z. Y., Walsh, S. R., et al. (2007). Non-invasive MR imaging of inflammation in a patient with both asymptomatic carotid atheroma and an abdominal aortic aneurysm: a case report. Annals of Surgical Innovation and Research, 1, 4.
Metz, S., Beer, A. J., Settles, M., Pelisek, J., Botnar, R. M., Rummeny, E. J., & Heider, P. (2011). Characterization of carotid artery plaques with USPIO-enhanced MRI: Assessment of inflammation and vascularity as in vivo imaging biomarkers for plaque vulnerability. International Journal of Cardiovascular Imaging, 27, 901–912.
Institutional Animal Care and Use Committee. (2011). Guide for the care and use of laboratory animals. Washington, D.C: The National Academies Press.
Constantinides, P., & Chakravarti, R. N. (1961). Rabbit arterial thrombosis production by systemic procedures. Archives of Pathology, 72, 197–208.
Chen, W. Q., Zhang, L., Liu, Y. F., Chen, L., Ji, X. P., Zhang, M., et al. (2007). Prediction of atherosclerotic plaque ruptures with high-frequency ultrasound imaging and serum inflammatory markers. American Journal of Physiology Heart and Circulatory Physiology, 293, H2836–H2844.
Yonemitsu, Y., Kaneda, Y., Tanaka, S., Nakashima, Y., Komori, K., Sugimachi, K., & Sueishi, K. (1998). Transfer of wild-type p53 gene effectively inhibits vascular smooth muscle cell proliferation in vitro and in vivo. Circulation Research, 82, 147–156.
Saam, T., Hatsukami, T. S., Takaya, N., Chu, B., Underhill, H., Kerwin, W. S., et al. (2007). The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology, 244, 64–77.
Trivedi, R. A., Mallawarachi, C., U-King-Im, J. M., Graves, M. J., Horsley, J., Goddard, M. J., et al. (2006). Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology, 26, 1601–1606.
Zhou, Q., Yang, K. R., Gao, P., Chen, W. L., Yang, D. Y., Liang, M. J., & Zhu, L. (2011). An experimental study on MR imaging of atherosclerotic plaque with SPIO marked endothelial cells in a rabbit model. Journal of Magnetic Resonance Imaging, 34, 1325–1332.
Makowski, M. R., Varma, G., Wiethoff, A. J., Smith, A., Mattock, K., Jansen, C. H., et al. (2011). Noninvasive assessment of atherosclerotic plaque progression in ApoE−/− mice using susceptibility gradient mapping. Circulation Cardiovascular Imaging, 4, 295–303.
Clarke, S. E., Beletsky, V., Hammond, R. R., Hegele, R. A., & Rutt, B. K. (2006). Validation of automatically classified magnetic resonance images for carotid plaque compositional analysis. Stroke, 37, 93–97.
Kooi, M. E., Cappendijk, V. C., Cleutjens, K. B., Kessels, A. G., Kitslaar, P. J., Borgers, M., et al. (2003). Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation, 107, 2453–2458.
Trivedi, R. A., U-King-Im, J. M., Graves, M. J., Cross, J. J., Horsley, J., Goddard, M. J., et al. (2004). In vivo detection of macrophages in human carotid atheroma: temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke, 35, 1631–1635.
Tabas, I., Williams, K. J., & Boren, J. (2007). Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation, 116, 1832–1844.
Moreno, P. R., Falk, E., Palacios, I. F., Newell, J. B., Fuster, V., & Fallon, J. T. (1994). Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation, 90, 775–778.
Acknowledgments
This study was supported by the grants for postdoctoral studies (No. 53431002 and China Postdoctoral Science Foundation No. 2012M512177) and Xuzhou Scientific Technology Grant (Nos. XF11C103 and XM13B047).
Author information
Authors and Affiliations
Corresponding author
Additional information
Chun-mei Qi, Lili Du and Ji Hao have contributed equally to this work and should be consider co-first authors.
Rights and permissions
About this article
Cite this article
Qi, Cm., Du, L., Wu, Wh. et al. Detection of Vulnerable Atherosclerotic Plaques in Experimental Atherosclerosis with the USPIO-Enhanced MRI. Cell Biochem Biophys 73, 331–337 (2015). https://doi.org/10.1007/s12013-015-0591-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-015-0591-y