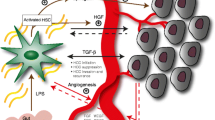
Abstract
Tumor cell microenvironment defines cancer development, also in hepatocellular carcinoma (HCC). Hepatic stellate cells (HSCs) are believed to be the key contributors to tumor microenvironment in HCC, yet their precise role in cancer progression is still unclear. The aim of this study was to determine the effect of human HSCs on progression of HCC using a subcutaneous xenograft nude mouse model. Nude mice were stratified to receive subcutaneous injections of human HCC cell line HepG2 and human HSC line LX-2 (HepG2 + LX-2), HepG2 alone, LX-2 alone, or phosphate-buffered saline. Tumor growth was assessed by measuring tumor size. After 30 days, final tumor size, weight, and histology were assessed. Compared with mice that were only injected HepG2 cells, mice injected with HepG2 + LX-2 exhibited more rapid tumor growth, increased tumor size and weight, higher tumor cell numbers due to increased proliferation and reduced apoptosis, increased fibrotic bands containing LX-2 cells, and increased tumor angiogenesis. In conclusion, HSCs play a significant role in promotion of HCC growth.












Similar content being viewed by others
Abbreviations
- HSCs:
-
Hepatic stellate cells
- HCC:
-
Hepatocellular carcinoma
- PCNA:
-
Proliferating cell nuclear antigen
References
Ferlay, J., Shin, H. R., Bray, F., Forman, D., Mathers, C., & Parkin, D. M. (2010). Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer, 127, 2893–2917.
Orimo, A., Gupta, P. B., Sgroi, D. C., Arenzana-Seisdedos, F., Delaunay, T., Naeem, R., et al. (2005). Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell, 121, 335–348.
Boire, A., Covic, L., Agarwal, A., Jacques, S., Sherifi, S., & Kuliopulos, A. (2005). PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell, 120, 303–313.
Tuxhorn, J. A., McAlhany, S. J., Dang, T. D., Ayala, G. E., & Rowley, D. R. (2002). Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Research, 62, 3298–3307.
Liotta, L. A., & Kohn, E. C. (2001). The microenvironment of the tumour-host interface. Nature, 411, 375–379.
Fidler, I. J. (2002). The organ microenvironment and cancer metastasis. Differentiation, 2002(70), 498–505.
De Wever, O., & Mareel, M. (2003). Role of tissue stroma in cancer cell invasion. The Journal of Pathology, 200, 429–447.
Friedman, S. L. (2004). Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nature Clinical Practice Gastroenterology and Hepatology, 1, 98–105.
Bataller, R., & Brenner, D. A. (2005). Liver fibrosis. The Journal of Clinical Investigation, 115, 209–218.
Jiao, J., Friedman, S. L., & Aloman, C. (2009). Hepatic fibrosis. Current Opinion in Gastroenterology, 25, 223–229.
Faouzi, S., Le, B. B., Neaud, V., Boussarie, L., Saric, J., Bioulac-Sage, P., et al. (1999). Myofibroblasts are responsible for collagen synthesis in the stroma of human hepatocellular carcinoma: An in vivo and in vitro study. The Journal of Hepatology, 30, 275–284.
Amann, T., Bataille, F., Spruss, T., Muhlbauer, M., Gabele, E., Scholmerich, J., et al. (2009). Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Science, 100, 646–653.
Friedman, S. L., Roll, F. J., Boyles, J., & Bissell, D. M. (1985). Hepatic lipocytes: The principal collagen-producing cells of normal rat liver. Proceedings of the National Academy of Sciences of the United States of America, 82, 8681–8685.
Wynn, T. A. (2008). Cellular and molecular mechanisms of fibrosis. The Journal of Pathology, 2008(214), 199–210.
Sokolovic, A., Sokolovic, M., Boers, W., Elferink, R. P., & Bosma, P. J. (2010). Insulin-like growth factor binding protein 5 enhances survival of LX2 human hepatic stellate cells. Fibrogenesis Tissue Repair, 3, 3.
Apte, M. V., Park, S., Phillips, P. A., Santucci, N., Goldstein, D., Kumar, R. K., et al. (2004). Desmoplastic reaction in pancreatic cancer: Role of pancreatic stellate cells. Pancreas, 29, 179–187.
Bachem, M. G., Schunemann, M., Ramadani, M., Siech, M., Beger, H., Buck, A., et al. (2005). Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology, 128, 907–921.
Hwang, R. F., Moore, T., Arumugam, T., Ramachandran, V., Amos, K. D., Rivera, A., et al. (2008). Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Research, 68, 918–926.
Pinzani, M., Milani, S., Grappone, C., Weber, F. L, Jr, Gentilini, P., & Abboud, H. E. (1994). Expression of platelet-derived growth factor in a model of acute liver injury. Hepatology, 19, 701–707.
Claesson-Welsh, L., Eriksson, A., Moren, A., Severinsson, L., Ek, B., Ostman, A., et al. (1988). cDNA cloning and expression of a human platelet-derived growth factor (PDGF) receptor specific for B-chain-containing PDGF molecules. Molecular and Cellular Biology, 8, 3476–3486.
Westermark, B., Siegbahn, A., Heldin, C. H., & Claesson-Welsh, L. (1990). B-type receptor for platelet-derived growth factor mediates a chemotactic response by means of ligand-induced activation of the receptor protein-tyrosine kinase. Proceedings of the National Academy of Sciences of the United States of America, 87, 128–132.
Lai, C. C., Henningson, C., & DiMaio, D. (1998). Bovine papillomavirus E5 protein induces oligomerization and trans-phosphorylation of the platelet-derived growth factor beta receptor. Proceedings of the National Academy of Sciences of the United States of America, 95, 15241–15246.
Friedman, S. L., & Arthur, M. J. (1989). Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. The Journal of Clinical Investigation, 84, 1780–1785.
Lee, J. S., Semela, D., Iredale, J., & Shah, V. H. (2007). Sinusoidal remodeling and angiogenesis: A new function for the liver-specific pericyte? Hepatology, 45, 817–825.
Olaso, E., Salado, C., Egilegor, E., Gutierrez, V., Santisteban, A., Sancho-Bru, P., et al. (2003). Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis. Hepatology, 37, 674–685.
Acknowledgments
Supported by National Natural Science Foundation of China (Grant Number 30971340 to Zhi-min Geng).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geng, Zm., Li, Qh., Li, Wz. et al. Activated Human Hepatic Stellate Cells Promote Growth of Human Hepatocellular Carcinoma in a Subcutaneous Xenograft Nude Mouse Model. Cell Biochem Biophys 70, 337–347 (2014). https://doi.org/10.1007/s12013-014-9918-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-9918-3