Abstract
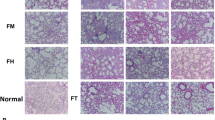
The purpose of the study was to explore the effects of breast regression protein 39 (BRP-39) in bleomycin-induced pulmonary fibrosis and its mechanism in pulmonary fibrosis by studying change in BRP-39 to provide a novel direction for the treatment of idiopathic pulmonary fibrosis. SPF grade male C57BL/6 rats were randomly divided into three groups, including bleomycin group, bleomycin+ BRP-39 recombinant protein group and control group. HE and Masson staining were applied to test the change in lung tissue after being treated by BRP-39, ELISA was applied to test the expression of TGF-β1 in different groups, and Western blot was used to test the expression of BRP-39 in rat lung tissue. Expression of BRP-39 increased, the fibrosis was obvious, and lung tissue collagen increased in bleomycin-induced pulmonary fibrosis in rat lung tissue. Increasing BRP-39 protein level and intratracheal bleomycin medication to establish pulmonary fibrosis model can aggravate pulmonary fibrosis. Along with the increase in BRP-39 protein level, TGF-β1 expression level also increased in lung tissue. Western blot results showed the expression of BRP-39, and TGF-β1 had the same trend in different groups. BRP-39 has effects in bleomycin-induced rat pulmonary fibrosis. Change in BRP-39 can affect the process of bleomycin-induced pulmonary fibrosis. The mechanism of BRP-3 in pulmonary fibrosis may work by regulating TGF-β1.






Similar content being viewed by others
References
Xu, K., Wang, L., Qiang, M., Li, P., & Tang, B. (2011). A selective near-infrared fluorescent probe for singlet oxygen in living cells. Chemical Communications (Cambridge, England), 47(26), 7386–7388.
Jones, M. G., Fletcher, S., & Richeldi, L. (2013). Idiopathic pulmonary fibrosis: Recent trials and current drug therapy. Respiration; international review of thoracic diseases, 68(5), 353–363.
Feng, R. E. (2013). Idiopathic pleuroparenchymal fibroelastosis: A new subtype of idiopathic interstitial pneumonia. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases, 36(5), 329–330.
Sato, A., Tabata, M., Hayashi, K., & Saruta, T. (2003). Effects of the angiotensin II type 1 receptor antagonist candesartan, compared with angiotensin-converting enzyme inhibitors, on the urinary excretion of albumin and type IV collagen in patients with diabetic nephropathy. Clinical and experimental nephrology, 7(3), 215–220.
Chou, H. C., Lang, Y. D., Wang, L. F., Wu, T. Y., Hsieh, Y. F., & Chen, C. M. (2012). Angiotensin II type 1 receptor antagonist attenuates lung fibrosis in hyperoxia-exposed newborn rats. The Journal of pharmacology and experimental therapeutics, 340(1), 169–175.
Furuhashi, K., Suda, T., Nakamura, Y., Inui, N., Hashimoto, D., Miwa, S., et al. (2010). Increased expression of YKL-40, a chitinase-like protein, in serum and lung of patients with idiopathic pulmonary fibrosis. Respiratory Medicine, 104(8), 1204–1210.
Korthagen, N. M., van Moorsel, C. H., Barlo, N. P., Ruven, H. J., Kruit, A., Heron, M., et al. (2011). Serum and BALF YKL-40 levels are predictors of survival in idiopathic pulmonary fibrosis. Respiratory Medicine, 105(1), 106–113.
Kastrup, J., Johansen, J. S., Winkel, P., Hansen, J. F., Hildebrandt, P., Jensen, G. B., et al. (2009). High serum YKL-40 concentration is associated with cardiovascular and all-cause mortality in patients with stable coronary artery disease. European Heart Journal, 30(9), 1066–1072.
Mygind, N. D., Iversen, K., Kober, L., Goetze, J. P., Nielsen, H., Boesgaard, S., et al. (2013). The inflammatory biomarker YKL-40 at admission is a strong predictor of overall mortality. Journal of Internal Medicine, 273(2), 205–216.
Lee, C. G., Hartl, D., Lee, G. R., Koller, B., Matsuura, H., Da Silva, C. A., et al. (2009). Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. The Journal of Experimental Medicine, 206(5), 1149–1166.
Nikota, J. K., Botelho, F. M., Bauer, C. M., Jordana, M., Coyle, A. J., Humbles, A. A., et al. (2011). Differential expression and function of breast regression protein 39 (BRP-39) in murine models of subacute cigarette smoke exposure and allergic airway inflammation. Respiratory Research, 12, 39.
Wcislo-Dziadecka, D., Kotulska, A., Brzezinska-Wcislo, L., Widuchowska, M., Lis-Swiety, A., Kopec-Medrek, M., et al. (2010). Serum human cartilage glycoprotein-39 in patients with systemic sclerosis: Relationship to skin and articular manifestation. Clinical Rheumatology, 29(8), 933–935.
Zhang, W., Murao, K., Zhang, X., Matsumoto, K., Diah, S., Okada, M., et al. (2010). Resveratrol represses YKL-40 expression in human glioma U87 cells. BMC Cancer, 10, 593.
Brochner, C. B., Johansen, J. S., Larsen, L. A., Bak, M., Mikkelsen, H. B., Byskov, A. G., et al. (2012). YKL-40 is differentially expressed in human embryonic stem cells and in cell progeny of the three germ layers. The Journal of Histochemistry and Cytochemistry, 60(3), 188–204.
Martinelli, M., Pacilli, A. M., Rivetti, S., Lauriola, M., Fasano, L., Carbonara, P., et al. (2011). A role for epidermal growth factor receptor in idiopathic pulmonary fibrosis onset. Molecular Biology Reports, 38(7), 4613–4617.
Li, X. X., Li, N., Ban, C. J., Zhu, M., Xiao, B., & Dai, H. P. (2011). Idiopathic pulmonary fibrosis in relation to gene polymorphisms of transforming growth factor-beta1 and plasminogen activator inhibitor 1. Chinese Medical Journal, 124(13), 1923–1927.
Kliment, C. R., & Oury, T. D. (2010). Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radical Biology & Medicine, 49(5), 707–717.
Rydell-Tormanen, K., Andreasson, K., Hesselstrand, R., Risteli, J., Heinegard, D., Saxne, T., et al. (2012). Extracellular matrix alterations and acute inflammation; developing in parallel during early induction of pulmonary fibrosis. Laboratory Investigation, 92(6), 917–925.
Shimbori, C., Gauldie, J., & Kolb, M. (2013). Extracellular matrix microenvironment contributes actively to pulmonary fibrosis. Current Opinion in Pulmonary Medicine, 19(5), 446–452.
Li, M., Krishnaveni, M. S., Li, C., Zhou, B., Xing, Y., Banfalvi, A., et al. (2011). Epithelium-specific deletion of TGF-beta receptor type II protects mice from bleomycin-induced pulmonary fibrosis. The Journal of Clinical Investigation, 121(1), 277–287.
Sureshbabu, A., Tonner, E., Allan, G. J., & Flint, D. J. (2011). Relative roles of TGF-beta and IGFBP-5 in idiopathic pulmonary fibrosis. Pulmonary Medicine, 2011, 517687.
Lepparanta, O., Sens, C., Salmenkivi, K., Kinnula, V. L., Keski-Oja, J., Myllarniemi, M., et al. (2012). Regulation of TGF-beta storage and activation in the human idiopathic pulmonary fibrosis lung. Cell and Tissue Research, 348(3), 491–503.
Zhang, X., Zhang, Y., Tao, B., Teng, L., Li, Y., Cao, R., et al. (2012). Loss of Shp2 in alveoli epithelia induces deregulated surfactant homeostasis, resulting in spontaneous pulmonary fibrosis. FASEB Journal, 26(6), 2338–2350.
Neunlist, M., Aubert, P., Bonnaud, S., Van Landeghem, L., Coron, E., Wedel, T., et al. (2007). Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. American Journal of Physiology. Gastrointestinal and Liver Physiology, 292(1), G231–G241.
Kasai, H., Allen, J. T., Mason, R. M., Kamimura, T., & Zhang, Z. (2005). TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respiratory Research, 6, 56.
Lee, J., Choi, J. H., & Joo, C. K. (2013). TGF-beta1 regulates cell fate during epithelial-mesenchymal transition by upregulating survivin. Cell Death and Disease, 4, e714.
Doerner, A. M., & Zuraw, B. L. (2009). TGF-beta1 induced epithelial to mesenchymal transition (EMT) in human bronchial epithelial cells is enhanced by IL-1beta but not abrogated by corticosteroids. Respiratory Research, 10, 100.
Vivar, R., Humeres, C., Ayala, P., Olmedo, I., Catalan, M., Garcia, L., et al. (2013). TGF-beta1 prevents simulated ischemia/reperfusion-induced cardiac fibroblast apoptosis by activation of both canonical and non-canonical signaling pathways. Biochimica et Biophysica Acta, 1832(6), 754–762.
Li, C., Qu, X., Xu, W., Qu, N., Mei, L., Liu, Y., et al. (2013). Arsenic trioxide induces cardiac fibroblast apoptosis in vitro and in vivo by up-regulating TGF-beta1 expression. Toxicology Letters, 219(3), 223–230.
Son, J. Y., Kim, S. Y., Cho, S. H., Shim, H. S., Jung, J. Y., Kim, E. Y., et al. (2013). TGF-beta1 T869C polymorphism may affect susceptibility to idiopathic pulmonary fibrosis and disease severity. Lung, 191(2), 199–205.
Cu, A., Ye, Q., Sarria, R., Nakamura, S., Guzman, J., & Costabel, U. (2009). N-acetylcysteine inhibits TNF-alpha, sTNFR, and TGF-beta1 release by alveolar macrophages in idiopathic pulmonary fibrosis in vitro. Sarcoidosis, Vasculitis, and Diffuse Lung Diseases, 26(2), 147–154.
Kang, H. R., Cho, S. J., Lee, C. G., Homer, R. J., & Elias, J. A. (2007). Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. The Journal of biological chemistry, 282(10), 7723–7732.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, C., Yang, Y., Lin, Y. et al. Expression and Mechanism of BRP-39 in Bleomycin-Induced Pulmonary Fibrosis in Rat. Cell Biochem Biophys 70, 251–257 (2014). https://doi.org/10.1007/s12013-014-9889-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-9889-4