Abstract
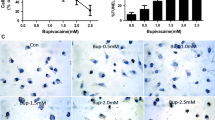
Bupivacaine is a sodium channel blocker, which is widely used for local infiltration nerve block, epidural and intrathecal anesthesia. However, bupivacaine could cause nerve damage. Hispidulin was shown to be able to penetrate the blood–brain barrier and possess antiepileptic activity. In this study, we investigate whether hispidulin administration could attenuate bupivacaine-induced neurotoxicity. Bupivacaine-challenged mouse neuroblastoma N2a cells were treated with hispidulin. The neuron injury was assessed by examination of cell viability and apoptosis. The levels of activation of AMP-activated protein kinase (AMPK) signaling pathway were examined along with the effect of blocking AMPK signaling on cell viability in the presence of hispidulin and bupivacaine. Our results showed that Bupivacaine treatment significantly decreased cell viability and induced apoptosis. Treatment with hispidulin significantly attenuated bupivacaine-induced cell injury. In addition, hispidulin treatment increased the levels of phospho-AMPK and phospho-GSK3β and attenuated bupivacaine-induced loss in mitochondrial membrane potential. Furthermore, we found that blocking AMPK signaling pathway significantly abolished the cytoprotective effect of hispidulin against bupivacaine-induced cell injury. Our findings suggest that treatment of neuroblastoma cells with hispidulin-protected neural cells from Bupivacaine-induced injury via the activation of the AMPK/GSK3β signaling pathway.






Similar content being viewed by others
References
Kohase, H., Miyamoto, T., & Umino, M. (2002). A new method of continuous maxillary nerve block with an indwelling catheter. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics, 94(2), 162–166.
Yang, S., et al. (2011). Local anesthetic Schwann cell toxicity is time and concentration dependent. Regional Anesthesia and Pain Medicine, 36(5), 444–451.
Hadzic, A., et al. (2004). A comparison of infraclavicular nerve block versus general anesthesia for hand and wrist day-case surgeries. Anesthesiology, 101(1), 127–132.
Liu, S. S., et al. (2005). A comparison of regional versus general anesthesia for ambulatory anesthesia: A meta-analysis of randomized controlled trials. Anesthesia and Analgesia, 101(6), 1634–1642.
Richman, J. M., et al. (2006). Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesthesia and Analgesia, 102(1), 248–257.
Perez-Castro, R., et al. (2009). Cytotoxicity of local anesthetics in human neuronal cells. Anesthesia and Analgesia, 108(3), 997–1007.
Koff, M. D., et al. (2008). Severe brachial plexopathy after an ultrasound-guided single-injection nerve block for total shoulder arthroplasty in a patient with multiple sclerosis. Anesthesiology, 108(2), 325–328.
Shah, S., et al. (2005). Neurologic complication after anterior sciatic nerve block. Anesthesia and Analgesia, 100(5), 1515–1517. (Table of contents).
Watts, S. A., & Sharma, D. J. (2007). Long-term neurological complications associated with surgery and peripheral nerve blockade: Outcomes after 1065 consecutive blocks. Anaesthesia and Intensive Care, 35(1), 24–31.
Hardie, D. G. (2007). AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nature Reviews Molecular Cell Biology, 8(10), 774–785.
Russell, R, 3rd. (2003). The Role of AMP-activated protein kinase in fuel selection by the stressed heart. Current Hypertension Reports, 5(6), 459–465.
Lee, S. J., et al. (2010). AMPK attenuates bupivacaine-induced neurotoxicity. Journal of Dental Research, 89(8), 797–801.
Bourdillat, B., et al. (1988). Mechanism of action of hispidulin, a natural flavone, on human platelets. Progress in Clinical and Biological Research, 280, 211–214.
He, L., et al. (2011). Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Science, 102(1), 219–225.
Tan, R. X., et al. (1999). Mono- and sesquiterpenes and antifungal constituents from Artemisia species. Planta Medica, 65(1), 64–67.
Kavvadias, D., et al. (2003). Constituents of sage (Salvia officinalis) with in vitro affinity to human brain benzodiazepine receptor. Planta Medica, 69(2), 113–117.
Kavvadias, D., et al. (2004). The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood–brain barrier and exhibits anticonvulsive effects. British Journal of Pharmacology, 142(5), 811–820.
Lin, Y. C., et al. (2010). Hispidulin potently inhibits human glioblastoma multiforme cells through activation of AMP-activated protein kinase (AMPK). Journal of Agriculture and Food Chemistry, 58(17), 9511–9517.
Khan, M., et al. (2012). Pseudolaric acid B induces caspase-dependent and caspase-independent apoptosis in u87 glioblastoma cells. Evidence Based Complementary an Alternative Medicine, 2012, 957568.
Lu, Y., et al. (2010). Anesthetic sevoflurane causes neurotoxicity differently in neonatal naive and Alzheimer disease transgenic mice. Anesthesiology, 112(6), 1404–1416.
Wei, H., et al. (2005). Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Research, 1037(1–2), 139–147.
Wang, X., et al. (2010). Alpha-lipoic acid prevents bupivacaine-induced neuron injury in vitro through a PI3K/Akt-dependent mechanism. Neurotoxicology, 31(1), 101–112.
Auroy, Y., et al. (1997). Serious complications related to regional anesthesia: Results of a prospective survey in France. Anesthesiology, 87(3), 479–486.
Johnson, M. E., et al. (2002). Effect of local anesthetic on neuronal cytoplasmic calcium and plasma membrane lysis (necrosis) in a cell culture model. Anesthesiology, 97(6), 1466–1476.
Ozcan, M., et al. (2010). Effects of levobupivacaine and bupivacaine on intracellular calcium signaling in cultured rat dorsal root ganglion neurons. Journal of Receptors Signal Transduction Research, 30(2), 115–120.
Lirk, P., et al. (2008). In vitro, inhibition of mitogen-activated protein kinase pathways protects against bupivacaine- and ropivacaine-induced neurotoxicity. Anesthesia and Analgesia, 106(5), 1456–1464. (table of contents).
Park, C. J., et al. (2005). Bupivacaine induces apoptosis via ROS in the Schwann cell line. Journal of Dental Research, 84(9), 852–857.
Ma, R., et al. (2010). Dexamethasone attenuated bupivacaine-induced neuron injury in vitro through a threonine-serine protein kinase B-dependent mechanism. Neuroscience, 167(2), 329–342.
Wang, Z., et al. (2012). Lithium attenuates bupivacaine-induced neurotoxicity in vitro through phosphatidylinositol-3-kinase/threonine-serine protein kinase B- and extracellular signal-regulated kinase-dependent mechanisms. Neuroscience, 206, 190–200.
Jung, J. E., et al. (2004). 5-Aminoimidazole-4-carboxamide-ribonucleoside enhances oxidative stress-induced apoptosis through activation of nuclear factor-kappaB in mouse Neuro 2a neuroblastoma cells. Neuroscience Letters, 354(3), 197–200.
Ramamurthy, S., & Ronnett, G. V. (2006). Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. Journal of Physiology, 574(Pt 1), 85–93.
Spasic, M. R., Callaerts, P., & Norga, K. K. (2009). AMP-activated protein kinase (AMPK) molecular crossroad for metabolic control and survival of neurons. Neuroscientist, 15(4), 309–316.
Lu, J., et al. (2011). Bupivacaine induces reactive oxygen species production via activation of the AMP-activated protein kinase-dependent pathway. Pharmacology, 87(3–4), 121–129.
Arsikin, K., et al. (2012). Autophagy-dependent and -independent involvement of AMP-activated protein kinase in 6-hydroxydopamine toxicity to SH-SY5Y neuroblastoma cells. Biochimica et Biophysica Acta, 1822(11), 1826–1836.
Culmsee, C., et al. (2001). AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. Journal of Molecular Neuroscience, 17(1), 45–58.
Kim, J., et al. (2011). AMPK activation inhibits apoptosis and tau hyperphosphorylation mediated by palmitate in SH-SY5Y cells. Brain Research, 1418, 42–51.
Mayer, C. M., & Belsham, D. D. (2010). Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: Rescue of resistance and apoptosis through adenosine 5′ monophosphate-activated protein kinase activation. Endocrinology, 151(2), 576–585.
Blazquez, C., et al. (2001). The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Letters, 489(2–3), 149–153.
Park, Y. J., et al. (2013). Activation of AMP-activated protein kinase alleviates homocysteine-mediated neurotoxicity in SH-SY5Y cells. Neurochemical Research, 38(8), 1561–1571.
Lu, J., et al. (2010). Quercetin activates AMP-activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol-induced neurotoxicity. The Journal of Pathology, 222(2), 199–212.
Zhang, X., et al. (2010). A pharmacological activator of AMP-activated protein kinase protects hypoxic neurons in a concentration-dependent manner. Neurochemical Research, 35(8), 1281–1289.
Su, C. C., et al. (2007). Phosphatidylinositol 3-kinase/Akt activation by integrin–tumor matrix interaction suppresses Fas-mediated apoptosis in T cells. The Journal of Immunology, 179(7), 4589–4597.
Nepal, M., et al. (2013). Hispidulin attenuates bone resorption and osteoclastogenesis via the RANKL-induced NF-κB and NFATc1 pathways. European Journal of Pharmacology, 715(1–3), 96–104.
Voelckel, W. G., et al. (2005). Signs of inflammation after sciatic nerve block in pigs. Anesthesia and Analgesia, 101(6), 1844–1846.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xinhuan Niu and Jie Chen have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Niu, X., Chen, J., Wang, P. et al. The Effects of Hispidulin on Bupivacaine-Induced Neurotoxicity: Role of AMPK Signaling Pathway. Cell Biochem Biophys 70, 241–249 (2014). https://doi.org/10.1007/s12013-014-9888-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-9888-5