Abstract
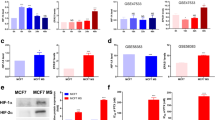
Mesenchymal stem cells (MSCs) have been shown to be able to inhibit cancer cells growth. In this study, we investigate the role and the molecular mechanism of hypoxia-inducible factor 1-alpha (HIF-1α) in inhibition of cancer cell proliferation by human MSCs through depletion and overexpression of HIF-1α in human MSCs. We found that the cell culture medium from HIF-1α-depleted Z3 cells significantly promotes breast cancer MCF-7 cell proliferation and colony formation. The expression of p21 is increased in MCF-7 cells, but p53 level remains unchanged. In contrast, the cultured medium from HIF-1α-overexpressed Z3 cells dramatically inhibits MCF-7 cell proliferation and colony formation. The expression of p21 is inhibited in MCF-7 cells, but p53 does not change. We conclude HIF-1α promotes inhibitory effect of human MCSs on breast cancer cell proliferation and colony formation. This process is tightly correlated with cell cycle protein p21 level in cancer cells.




Similar content being viewed by others
References
Khakoo, A. Y., Pati, S., Anderson, S. A., Reid, W., Elshal, M. F., Rovira, I. I., et al. (2006). Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. Journal of Experimental Medicine, 203, 1235–1247.
Hsieh, T. C., Wijeratne, E. K., Liang, J. Y., Gunatilaka, A. L., & Wu, J. M. (2005). Differential control of growth, cell cycle progression, and expression of NF-kappaB in human breast cancer cells MCF-7, MCF-10A, and MDA-MB-231 by ponicidin and oridonin, diterpenoids from the chinese herb Rabdosia rubescens. Biochemical and Biophysical Research Communications, 337, 224–231.
Brogi, E., Schatteman, G., Wu, T., Kim, E. A., Varticovski, L., Keyt, B., & Isner, J. M. (1996). Hypoxia-induced paracrine regulation of vascular endothelial growth factor receptor expression. Journal of Clinical Investigation, 97, 469–476.
Janssen, H. L., Haustermans, K. M., Sprong, D., Blommestijn, G., Hofland, I., Hoebers, F. J., et al. (2002). HIF-1A, pimonidazole, and iododeoxyuridine to estimate hypoxia and perfusion in human head-and-neck tumors. International Journal of Radiation Oncology Biology Physics, 54, 1537–1549.
Kitajima, Y., & Miyazaki, K. (2013). The critical impact of HIF-1a on gastric cancer biology. Cancers (Basel), 5, 15–26.
Kelly, B. D., Hackett, S. F., Hirota, K., Oshima, Y., Cai, Z., Berg-Dixon, S., et al. (2003). Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circulation Research, 93, 1074–1081.
Huang, J. H., Lee, F. S., Pasha, T. L., Sammel, M. D., Karakousis, G., Xu, G., et al. (2010). Analysis of HIF-1a and its regulator, PHD2, in retroperitoneal sarcomas: clinico-pathologic implications. Cancer Biology & Therapy, 9, 303–311.
Oladipupo, S. S., Hu, S., Santeford, A. C., Yao, J., Kovalski, J. R., Shohet, R. V., et al. (2011). Conditional HIF-1 induction produces multistage neovascularization with stage-specific sensitivity to VEGFR inhibitors and myeloid cell independence. Blood, 117, 4142–4153.
Kaidi, A., Williams, A. C., & Paraskeva, C. (2007). Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nature Cell Biology, 9, 210–217.
Tokita, K., Terasaki, P., Maruya, E., & Saji, H. (2001). Tumour regression following stem cell infusion from daughter to microchimeric mother. Lancet, 358, 2047–2048.
Maffioletti, S. M., Noviello, M., English, K., & Tedesco, F. S. (2014). Stem cell transplantation for muscular dystrophy: the challenge of immune response. Biomedical Research International, 2014, 964010.
Karam, C., Mauermann, M. L., Johnston, P. B., Lahoria, R., Engelstad, J. K., & Dyck, P. J. (2014). Immune-mediated neuropathies following stem cell transplantation. Journal of Neurology, Neurosurgery and Psychiatry, 85, 638–642.
Grayson, W. L., Zhao, F., Izadpanah, R., Bunnell, B., & Ma, T. (2006). Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. Journal of Cellular Physiology, 207, 331–339.
Engelhardt, S., Al-Ahmad, A. J., Gassmann, M., & Ogunshola, O. O. (2014). Hypoxia selectively disrupts brain microvascular endothelial tight junction complexes through a hypoxia-inducible factor-1 (HIF-1) dependent mechanism. Journal of Cellular Physiology, 229, 1096–1105.
Bosch, P., Pratt, S. L., & Stice, S. L. (2006). Isolation, characterization, gene modification, and nuclear reprogramming of porcine mesenchymal stem cells. Biology of Reproduction, 74, 46–57.
Zhao, L. R., Duan, W. M., Reyes, M., Keene, C. D., Verfaillie, C. M., & Low, W. C. (2002). Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Experimental Neurology, 174, 11–20.
Lee, J. Y., Choi, J. Y., Lee, K. M., Park, S. K., Han, S. H., Noh, D. Y., et al. (2008). Rare variant of hypoxia-inducible factor-1alpha (HIF-1A) and breast cancer risk in Korean women. Clinica Chimica Acta, 389, 167–170.
Nakatsuka, A., Wada, J., Hida, K., Hida, A., Eguchi, J., Teshigawara, S., et al. (2012). RXR antagonism induces G0/G1 cell cycle arrest and ameliorates obesity by up-regulating the p53-p21(Cip1) pathway in adipocytes. Journal of Pathology, 226, 784–795.
Yang, X., Wang, W., Qin, J. J., Wang, M. H., Sharma, H., Buolamwini, J. K., et al. (2012). JKA97, a novel benzylidene analog of harmine, exerts anti-cancer effects by inducing G1 arrest, apoptosis, and p53-independent up-regulation of p21. PLoS ONE, 7, e34303.
Wu, G., Lin, N., Xu, L., Liu, B., & Feitelson, M. A. (2013). UCN-01 induces S and G2/M cell cycle arrest through the p53/p21(waf1) or CHK2/CDC25C pathways and can suppress invasion in human hepatoma cell lines. BMC Cancer, 13, 167.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yuan Hu and Jing Bai have contributed equally to the work.
Rights and permissions
About this article
Cite this article
Hu, Y., Bai, J., Hou, SX. et al. Hypoxia-Inducible Factor 1-Alpha Regulates Cancer-Inhibitory Effect of Human Mesenchymal Stem Cells. Cell Biochem Biophys 72, 131–136 (2015). https://doi.org/10.1007/s12013-014-0420-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0420-8