Abstract
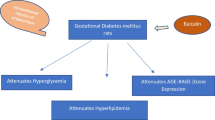
To investigate the protective effect of secretory receptor for advanced glycation endproducts (sRAGE) on the fetal development using rat model of gestational diabetes mellitus (GDM). The model of pregnant rats with intrauterine hyperglycemia was established by intraperitoneal injection of 25 mg/kg streptozotocin (STZ). Rats with established GDM were randomly grouped, and the pregnant rats in the experimental group were subsequently injected with recombinant sRAGE protein (5 mg/kg, in 0.2 mL PBS) at tail vein every 24 h, while the rats in control group were injected with the same dosage of albumin solution. Blood glucose, serum levels of advanced glycation endproducts (AGEs), and levels of RAGE protein in brain and heart tissues of pregnant rats were measured at 3, 13, and 19 days postconception. At 19 days fetuses were delivered by cesarean section, number of fetuses, their weight and placental weights were recorded, and fetal malformations and defects were analyzed visually and pathologically. The expression level of RAGE, NOX2, MCP-1, p65, VCAM-1, and VEGF mRNA in placenta was evaluated by real-time PCR. p65 protein localization was detected by immunohistochemistry in fetal brain and heart tissue sections. We analyzed the correlation between AGEs and RAGE level and the development of fetal rats, and the protective effect of blocking AGEs–RAGE pathway on the fetal development in the rat model of GDM was investigated. (1) The concentration of blood glucose and AGEs in serum of pregnant rats with GDM was significantly higher than in control group (p < 0.05), with strong correlation between blood glucose and levels of AGEs (r = 0.693, p < 0.05). (2) While both the number of fetuses and placental wet weight in pregnant rat model of GDM were similar to control group, pups from GDM group exhibited higher incidence of developmental abnormalities and higher average weight (p < 0.05). sRAGE treatment slightly but not significantly reduced the probability of the fetal developmental defects, as compared to GDM group. (3) p65, a part of the NF-kB heterodimeric complex, was localized to cell nuclei in the fetal tissues of pups delivered by GDM rats, while sRAGE treatment partially restored cytoplasmic localization of p65, similarly to control tissues. Increased incidence of fetal developmental defects observed in offsprings of pregnant rats with GDM had significant correlation with the level of AGEs in serum of pregnant rats and expression levels of RAGE protein in tissues. GDM resulted in upregulation of mRNA expression of several pro-inflammatory and ROS-inducing genes in placental tissues of pregnant rats. Elevated blood glucose, serum AGEs levels, and increased gene expression are attenuated by intravenous sRAGE treatment. sRAGE appears to reduce the activity of NF-κB in fetal tissues, thus potentially having a protective effect on fetal development.



Similar content being viewed by others
References
Imam, K. (2012). Gestational diabetes mellitus. Advances in Experimental Medicine and Biology, 771, 24–34.
Crowther, C. A., Hiller, J. E., Moss, J. R., McPhee, A. J., Jeffries, W. S., Robinson, J. S., et al. (2005). Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine, 352, 2477–2486.
Chawla, D., Bansal, S., Banerjee, B. D., Madhu, S. V., Kalra, O. P., & Tripathi, A. K. (2014). Role of advanced glycation end product (AGE)-induced receptor (RAGE) expression in diabetic vascular complications. Microvascular Research, 28(95C), 1–6.
Hu, H., Jiang, H., Ren, H., Hu, X., Wang, X., & Han, C. (2014). AGEs and chronic subclinical inflammation in diabetes: Disorders of immune system. Diabetes/Metabolism Research and Reviews,. doi:10.1002/dmrr.2560.
Yamagishi, S., & Matsui, T. (2010). Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxidative Medicine and Cellular Longevity, 3(2), 101–108.
de Ranitz-Greven, W. L., Kaasenbrood, L., Poucki, W. K., Hamerling, J., Bos, D. C., Visser, G. H., et al. (2012). Advanced glycation end products, measured as skin autofluorescence, during normal pregnancy and pregnancy complicated by diabetes mellitus. Diabetes Technology & Therapeutics, 14, 1134–1139.
Mai, C., Wang, B., Wen, J., Lin, X., & Niu, J. (2014). Lipoprotein-associated phospholipase A2 and AGEs are associated with cardiovascular risk factors in women with history of gestational diabetes mellitus. Gynecological Endocrinology: The Official Journal of the International Society of Gynecological Endocrinology, 30, 241–244.
Kalea, A. Z., Schmidt, A. M., & Hudson, B. I. (2011). Alternative splicing of RAGE: Roles in biology and disease. Front iers in Bioscience (Landmark Ed)., 1(16), 2756–2770.
Goh, S. Y., & Cooper, M. E. (2008). Clinical review: The role of advanced glycation end products in progression and complications of diabetes. The Journal of Clinical Endocrinology and Metabolism, 93, 1143–1152.
Guosheng, L., Hongmei, S., Chuan, N., Haiying, L., Xiaopeng, Z., & Xianqiong, L. (2009). The relationship of serum AGE levels in diabetic mothers with adverse fetal outcome. Journal of Perinatology: Official Journal of the California Perinatal Association, 29, 483–488.
Sukumar, P., Viswambharan, H., Imrie, H., Cubbon, R. M., Yuldasheva, N., Gage, M., et al. (2013). Nox2 NADPH oxidase has a critical role in insulin resistance-related endothelial cell dysfunction. Diabetes, 62(6), 2130–2134.
Panee, J. (2012). Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine, 60(1), 1–12.
Benter, I. F., Benboubetra, M., Hollins, A. J., Yousif, M. H., Canatan, H., & Akhtar, S. (2009). Early inhibition of EGFR signaling prevents diabetes-induced up-regulation of multiple gene pathways in the mesenteric vasculature. Vascular Pharmacology, 51(4), 236–245.
Gustavsson, C., Agardh, C. D., Zetterqvist, A. V., Nilsson, J., Agardh, E., & Gomez, M. F. (2010). Vascular cellular adhesion molecule-1 (VCAM-1) expression in mice retinal vessels is affected by both hyperglycemia and hyperlipidemia. PLoS One, 5(9), e12699.
Chen, P., Chen, J. B., Chen, W. Y., Zheng, Q. L., Wang, Y. Q., & Xu, X. J. (2012). Effects of quercetin on nuclear factor-κB p65 expression in renal ubiquitin-proteasome system of diabetic rats. Zhonghua Nei Ke Za Zhi, 51(6), 460–465.
Devaraj, S., Cheung, A. T., Jialal, I., et al. (2007). Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes, 56, 2790–2796.
Hofmann, M. A., Schiekofer, S., Isermann, B., et al. (1999). Peripheral blood mononuclear cells isolated from patients with diabetic nephropathy show increased activation of the oxidative-stress sensitive transcription factor NF-kappaB. Diabetologia, 42, 222–232.
Pedula, K. L., Hillier, T. A., Schmidt, M. M., et al. (2009). Ethnic differences in gestational oral glucose screening in a large US population. Ethnicity and Disease, 19, 414–419.
Page, K. A., Romero, A., Buchanan, T. A., & Xiang, A. H. (2014). Gestational diabetes mellitus, maternal obesity, and adiposity in offspring. The Journal of Pediatrics, 164, 807–810.
Buchanan, T. A., & Xiang, A. H. (2005). Gestational diabetes mellitus[J]. The Journal of Clinical Investigation, 115(3), 485–491.
Kjos, S. L., & Buchanan, T. A. (1999). Gestational diabetes mellitus. New England Journal of Medicine, 341(23), 1749–1756.
Jovanovic, L., & Pettitt, D. J. (2001). Gestational diabetes mellitus. JAMA, 286(20), 2516–2518.
Coustan, D. R. (2013). Gestational diabetes mellitus. Clinical Chemistry, 59(9), 1310–1321.
Gilmartin, A. B., Ural, S. H., & Repke, J. T. (2007). Gestational diabetes mellitus. Reviews in Obstetrics and Gynecology, 1(3), 129–134.
Landon, M. B., & Gabbe, S. G. (2011). Gestational diabetes mellitus. Obstetrics and Gynecology, 118(6), 1379–1393.
Wei, Y. M., & Yang, H. X. (2011). Comparison of the diagnostic criteria for gestational diabetes mellitus in China. Zhonghua fu chan ke za zhi, 46, 578–581.
Rong, L. L., Yan, S. F., Wendt, T., Hans, D., Pachydaki, S., Bucciarelli, L. G., et al. (2004). RAGE modulates peripheral nerve regeneration via recruitment of both inflammatory and axonal outgrowth pathways. FASEB, 18, 1818–1825.
Yonekura, H., Yamamoto, Y., Sakurai, S., Petrova, R. G., Abedin, M. J., Li, H., et al. (2003). Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochemical Journal, 370, 1097–1109.
Huttunen, H. J., Fages, C., & Rauvala, H. (1999). Receptor for advanced glycation end-products (RAGE)—Mediated neurite outgrowth and activation of NF-kB require the cytoplasmic domain of the receptor but different downstream signaling pathways. Journal of Biological Chemistry, 274, 19919–19924.
Yan, S. D., Schmidt, A. M., Anderson, G. M., Zhang, J., Brett, J., Zou, Y. S., et al. (1994). Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. Journal of Biological Chemistry, 269, 9889–9897.
Basta, G., Lazzerini, G., Massaro, M., Simoncini, T., Tanganelli, P., Fu, C., et al. (2002). Advanced glycation end-products activate endothelium through signal-transduction receptor RAGE: A mechanism for amplification of inflammatory responses. Circulation, 105, 816–822.
Wautier, M. P., Chappey, O., Corda, S., Stern, D. M., Schmidt, A. M., & Wautier, J. L. (2001). Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. The American Journal of Physiology: Endocrinology and Metabolism, 280, E685–E694.
Alberti, K. G. M. M., & Zimmet, P. Z. (1998). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine, 15(7), 539–553.
Consultation, W. H. O. (1999). Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xuwen Tang and Qingxin Qin have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Tang, X., Qin, Q., Xie, X. et al. Protective Effect of sRAGE on Fetal Development in Pregnant Rats with Gestational Diabetes Mellitus. Cell Biochem Biophys 71, 549–556 (2015). https://doi.org/10.1007/s12013-014-0233-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0233-9