Abstract
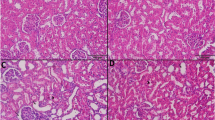
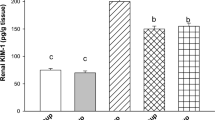
Cisplatin-induced generation of reactive oxygen species leads to acute nephrotoxicity limiting its use in the treatment of various cancers. Gelsemine, an alkaloid isolated from Gelsemium elegans, is known to possess anti-inflammatory and anti-cancer activities. This study was aimed to investigate as to whether gelsemine can serve as a protective agent against cisplatin-induced nephrotoxicity. Male Wistar rats were divided into 6 groups, each with 6 rats. Group 1 served as control and received the vehicles (peanut oil for 14 days and 0.9 % saline on day 14 for gelsemine and cisplatin respectively). Group 2 received a single intraperitoneal injection of cisplatin on day 14. Group 3 and 4 were pretreated with two different doses of gelsemine in addition to cisplatin, and group 5 and 6 received only gelsemine. The effects of gelsemine on cisplatin-induced nephrotoxicity were examined by measuring anti-oxidant enzymes activities, lipid peroxidation, and DNA damage in the kidneys, a well-established model of oxidative damage. Pretreatment of rats with gelsemine caused a significant attenuation of cisplatin-induced DNA and oxidative damages. The blockade of lipid peroxidation and xanthine oxidase activity was accompanied by increased production and/or activity of anti-oxidants, both enzymatic (catalase, glutathione peroxidase, glutathione reductase, and glutathione-S-transferase) and non-enzymatic (GSH). The biomarkers of kidney malfunctioning, creatinine, and blood urea nitrogen were ameliorated. The results of the present study suggest that gelsemine effectively suppressed cisplatin-induced renal injury by improving redox status.




Similar content being viewed by others
References
Sultana, S., Verma, K., & Khan, R. (2012). Nephroprotective efficacy of chrysin against cisplatin-induced toxicity via attenuation of oxidative stress. Journal of Pharmacy and Pharmacology, 64, 872–881.
Sanatani, M. S., Lazo-Langner, A., & Al-Rasheedy, I. M. (2013). Cisplatin and short-term 5-Fluorouracil infusion for paraneoplastic microangiopathic hemolytic anemia in gastric cancer: a case report and review of the literature. Case Reports in Oncological Medicine, 2013, 594787.
Petrelli, F., Zaniboni, A., Coinu, A., et al. (2013). Cisplatin or not in advanced gastric cancer: a systematic review and meta-analysis. PLoS ONE, 8, e83022.
Zhang, G., Fu, C., Zhang, Y., et al. (2012). Extended-field intensity-modulated radiotherapy and concurrent cisplatin-based chemotherapy for postoperative cervical cancer with common iliac or para-aortic lymph node metastases: a retrospective review in a single institution. International Journal of Gynecological Cancer, 22, 1220–1225.
Jiang, L., Yang, K. H., Guan, Q. L., et al. (2012). Cisplatin plus etoposide versus other platin-based regimens for patients with extensive small-cell lung cancer: a systematic review and meta-analysis of randomised, controlled trials. The Internal Medicine Journal, 42, 1297–1309.
Marullo, R., Werner, E., Degtyareva, N., Moore, B., Altavilla, G., Ramalingam, S. S., et al. (2013). Cisplatin induces mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redo status and bioenergetic functions. PLoS ONE, 8(11), e81162.
Yang, Y., Liu, H., Liu, F., & Dong, Z. (2014). Mitochondrial dysregulation and protection in cisplatin nephrotoxicity. Archives of Toxicology, 88, 1249–1256.
Waly, M. I., Ali, B. H., & Nemmar, A. (2013). Acute effects of diesel exhaust particles and cisplatin on oxidative stress in cultured human kidney (HEK 293) cells, and the influence of curcumin thereon. Toxicology in Vitro, 27, 2299–2304.
Domitrovic, R., Cvijanovic, O., Pernjak-Pugel, E., et al. (2013). Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food and Chemical Toxicology, 62, 397–406.
Waly, M. I., Ali, B. H., Al-Lawati, I., et al. (2013). Protective effects of emodin against cisplatin-induced oxidative stress in cultured human kidney (HEK 293) cells. Journal of Applied Toxicology, 33, 626–630.
Waly, M. I., Al Moundhri, M. S., & Ali, B. H. (2011). Effect of curcumin on cisplatin- and oxaliplatin-induced oxidative stress in human embryonic kidney (HEK) 293 cells. Renal Failure, 33, 518–523.
Rodrigues, M. A., Rodrigues, J. L., Martins, N. M., et al. (2011). Carvedilol protects against cisplatin-induced oxidative stress, redox state unbalance and apoptosis in rat kidney mitochondria. Chemico-Biological Interactions, 189, 45–51.
Rjiba-Touati, K., Boussema, I. A., Belarbia, A., et al. (2011). Protective effect of recombinant human erythropoietin against cisplatin-induced oxidative stress and nephrotoxicity in rat kidney. International Journal of Toxicology, 30, 510–517.
Dogukan, A., Tuzcu, M., Agca, C. A., et al. (2011). A tomato lycopene complex protects the kidney from cisplatin-induced injury via affecting oxidative stress as well as Bax, Bcl-2, and HSPs expression. Nutrition and Cancer, 63, 427–434.
Liao, Y. J., Jin, Y. P., Lin, L., et al. (2010). Determination of oxidative damage on DNA in brain and kidney of mice induced by anti-tumor agent of cisplatin. Zhongguo Ying Yong Sheng Li Xue Za Zhi, 26, 180–181.
Santos, N. A., Bezerra, C. S., Martins, N. M., et al. (2008). Hydroxyl radical scavenger ameliorates cisplatin-induced nephrotoxicity by preventing oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Cancer Chemotherapy and Pharmacology, 61, 145–155.
Santos, N. A., Catao, C. S., Martins, N. M., et al. (2007). Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Archives of Toxicology, 81, 495–504.
Dillioglugil, M. O., Maral Kir, H., Gulkac, M. D., et al. (2005). Protective effects of increasing vitamin E and a doses on cisplatin-induced oxidative damage to kidney tissue in rats. Urologia Internationalis, 75, 340–344.
Liu, M., Shen, J., Liu, H., et al. (2011). Gelsenicine from Gelsemium elegans attenuates neuropathic and inflammatory pain in mice. Biological and Pharmaceutical Bulletin, 34, 1877–1880.
Liu, M., Huang, H. H., Yang, J., et al. (2013). The active alkaloids of Gelsemium elegans Benth. are potent anxiolytics. Psychopharmacology (Berl), 225, 839–851.
Zhang, J.-Y., Gong, N., Huang, J.-L., Guo, L.-C., & Wan, Y.-X. (2013). Gelsemine, a principal alkaloid from Gelsemium sempervirens Ait., exhibits potent and specific antinociception in chronic pain by acting at spinal a3 glycine receptors. Pain, 154, 2452–2462.
Small, D. M., Bennett, N. C., Roy, S., et al. (2012). Oxidative stress and cell senescence combine to cause maximal renal tubular epithelial cell dysfunction and loss in an in vitro model of kidney disease. Nephron Experimental Nephrology, 122, 123–130.
de Munck, E., Munoz-Saez, E., Antonio, M. T., et al. (2013). Effect of beta-N-methylamino-l-alanine on oxidative stress of liver and kidney in rat. Environmental Toxicology and Pharmacology, 35, 193–199.
Annavarajula, S. K., Dakshinamurty, K. V., Naidu, M. U., et al. (2012). The effect of l-arginine on arterial stiffness and oxidative stress in chronic kidney disease. The Indian Journal of Nephrology, 22, 340–346.
Balat, A., Resic, H., Bellinghieri, G., et al. (2012). Devil’s Triangle in Kidney Diseases: Oxidative Stress, Mediators, and Inflammation. The International Journal of Nephrology, 2012, 156286.
Bhattacharya, S., Manna, P., Gachhui, R., et al. (2013). d-saccharic acid 1,4-lactone protects diabetic rat kidney by ameliorating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via NF-kappaB and PKC signaling. Toxicology and Applied Pharmacology, 267, 16–29.
Park, C. H., Lee, S. L., Okamoto, T., et al. (2012). Rokumi-jio-gan-Containing Prescriptions Attenuate Oxidative Stress, Inflammation, and Apoptosis in the Remnant Kidney. Evidence-Based Complementary and Alternative Medicine, 2012, 587902.
Chargui, I., Grissa, I., Bensassi, F., et al. (2012). Oxidative stress, biochemical and histopathological alterations in the liver and kidney of female rats exposed to low doses of deltamethrin (DM): A molecular assessment. Biomedical and Environmental Sciences, 25, 672–683.
Ghosh, P., Roy, S. S., Chakraborty, P., et al. (2013). Effects of organoselenium compound 2-(5-selenocyanato-pentyl)-benzo[de]isoquinoline 1,3-dione on cisplatin induced nephrotoxicity and genotoxicity: An investigation of the influence of the compound on oxidative stress and antioxidant enzyme system. BioMetals, 26, 61–73.
Kimoto, Y., Nishinohara, M., Sugiyama, A., et al. (2013). Protective effect of lactoferrin on Cisplatin-induced nephrotoxicity in rats. Journal of Veterinary Medical Science, 75, 159–164.
Mahmoud, M. F., & El Shazly, S. M. (2013). Pioglitazone protects against cisplatin induced nephrotoxicity in rats and potentiates its anticancer activity against human renal adenocarcinoma cell lines. Food and Chemical Toxicology, 51, 114–122.
Kong, D., Zhuo, L., Gao, C., et al. (2013). Erythropoietin protects against cisplatin-induced nephrotoxicity by attenuating endoplasmic reticulum stress-induced apoptosis. Journal of Nephrology, 26, 219–227.
Ali, B. H., Al-Salam, S., Al Husseini, I. S., et al. (2013). Abrogation of cisplatin-induced nephrotoxicity by emodin in rats. Fundamental and Clinical Pharmacology, 27, 192–200.
Zhao, Q.-C., Hua, W., Zhang, L., Guo, T., Zhao, M.-H., Yan, M., et al. (2010). Antitumor activity of two gelsemine metabolites in rat liver microsomes. Journal of Asian Natural Products Research, 12(9), 731–739.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lin Lin and Jing Zheng have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Lin, L., Zheng, J., Zhu, W. et al. Nephroprotective Effect of Gelsemine Against Cisplatin-Induced Toxicity is Mediated Via Attenuation of Oxidative Stress. Cell Biochem Biophys 71, 535–541 (2015). https://doi.org/10.1007/s12013-014-0231-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0231-y