Abstract
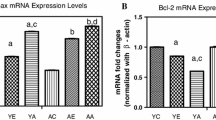
Cell phone radiation exposure and its biological interaction is the present concern of debate. Present study aimed to investigate the effect of 3G cell phone exposure with computer controlled 2-D stepper motor on 45-day-old male Wistar rat brain. Animals were exposed for 2 h a day for 60 days by using mobile phone with angular movement up to zero to 30°. The variation of the motor is restricted to 90° with respect to the horizontal plane, moving at a pre-determined rate of 2° per minute. Immediately after 60 days of exposure, animals were scarified and numbers of parameters (DNA double-strand break, micronuclei, caspase 3, apoptosis, DNA fragmentation, expression of stress-responsive genes) were performed. Result shows that microwave radiation emitted from 3G mobile phone significantly induced DNA strand breaks in brain. Meanwhile a significant increase in micronuclei, caspase 3 and apoptosis were also observed in exposed group (P < 0.05). Western blotting result shows that 3G mobile phone exposure causes a transient increase in phosphorylation of hsp27, hsp70, and p38 mitogen-activated protein kinase (p38MAPK), which leads to mitochondrial dysfunction-mediated cytochrome c release and subsequent activation of caspases, involved in the process of radiation-induced apoptotic cell death. Study shows that the oxidative stress is the main factor which activates a variety of cellular signal transduction pathways, among them the hsp27/p38MAPK is the pathway of principle stress response. Results conclude that 3G mobile phone radiations affect the brain function and cause several neurological disorders.









Similar content being viewed by others
References
Tillmann, T., Ernst, H., Streckert, J., Zhou, Y., Taugner, F., Hansen, V., et al. (2010). Indication of cocarcinogenic potential of chronic UMTS-modulated radiofrequency exposure in an ethylnitrosourea mouse model. International Journal of Radiation Biology, 86(7), 529–541.
Manti, L., Braselmann, H., Calabrese, M. L., Massa, R., Pugliese, M., Scampoli, P., et al. (2008). Effects of modulated microwave radiation at cellular telephone frequency (1.95 GHz) on X-ray-induced chromosome aberrations in human lymphocytes in vitro. Radiation Research, 169(5), 575–583.
Nora, D. V., Tomasi, D., Wang, G. J., Vaska, P., Fowler, J. S., Telang, F., et al. (2011). Effects of cell phone radiofrequency signal exposure on brain glucose metabolism. Journal of the American Medical Association, 305(8), 808–814.
Behari, J., & Nirala, J. P. (2012). SAR measurement due to mobile phone exposure in a simulated biological media. Electromagnetic Biology and Medicine, 31(3), 195–203.
Khurana, V. G., Teo, C., Kundi, M., Hardell, L., & Carlberg, M. (2009). Cell phones and brain tumors: A review including the long-term epidemiologic data. Surgical Neurology, 72, 205–215.
Kesari, K. K., Kumar, S., Nirala, J., Siddhiqui, M. H., & Behari, J. (2013). Biophysical evaluation of radiofrequency electromagnetic field effects on male reproductive pattern. Cell Biochemistry and Biophysics, 65(2), 85–96.
Mausset, A. L., de Seze, R., Montpeyroux, F., & Privat, A. (2001). Effects of radiofrequency exposure on the GABAergic system in the rat cerebellum: Clues fromsemi-quantitative immunohistochemistry. Brain Research, 912, 33–46.
Mausset-Bonnefont, A. L., Hirbec, H., Bonnefont, X., Privat, A., Vignon, J., & de Seze, R. (2004). Acute exposure to GSM 900-MHz electromagnetic fields induces glial reactivity and biochemical modifications in the rat brain. Neurobiology, 17, 445–454.
Odaci, E., Bas, O., & Kaplan, S. (2008). Effects of prenatal exposure to a 900 megahertz electromagnetic field on the dentate gyrus of rats: A stereological and histopathological study. Brain Research, 1238, 224–229.
Dimbylow, P. J., & Mann, S. M. (1994). SAR calculations in an anatomically realistic model of the head for mobile communication transceivers at 900 MHz and 1.8 GHz. Physical Medical Biology, 39, 1537–1544.
Rothman, K. J., Chou, C. K., Morgan, R., Balzano, Q., Guy, A. W., & Funch, D. P. (1996). Assessment of cellular telephone and other radio frequency exposure for epidemiologic research. Epidemiology, 7, 291–298.
Trunk, A., Stefanics, G., Zentai, N., Kovács-Bálint, Z., Thuróczy, G., & Hernádi, I. (2013). No effects of a single 3G UMTS mobile phone exposure on spontaneous EEG activity, ERP correlates, and automatic deviance detection. Bioelectromagnetics, 34(1), 31–42.
Danker-Hopfe, H., Dorn, H., Bahr, A., Anderer, P., & Sauter, C. (2011). Effects of electromagnetic fields emitted by mobile phones (GSM 900 and WCDMA/UMTS) on the macrostructure of sleep. Journal of Sleep Research, 20(1), 73–81.
Kesari, K. K., Kumar, S., & Behari, J. (2011). 900-MHz microwave radiation promotes oxidation in rat brain. Electromagnetic Biology and Medicine, 30(4), 219–234.
Kesari, K. K., Kumar, S., & Behari, J. (2012). Pathophysiology of microwave radiation: Effect on rat brain. Applied Biochemistry and Biotechnology, 166(2), 379–388.
Barth, A., Winker, R., Ponocny-Seliger, E., Mayrhofer, W., Ponocny, I., Sauter, C., et al. (2008). A meta-analysis for neurobehavioural effects due to electromagnetic field exposure emitted by GSM mobile phones. Occupational Environment and Medicine, 65(5), 342–346.
Kesari, K. K., Siddiqui, M. H., Meena, R., Verma, H. N., & Kumar, S. (2013). Cell phone radiation exposure on brain and associated biological systems. Indian Journal of Experimental Biology, 51(3), 187–200.
Kesari, K. K., Behari, J., & Kumar, S. (2010). Mutagenic response of 2.45 GHz radiation exposure on rat brain. International Journal of Radiation Biology, 86(4), 334–343.
Paulraj, R., & Behari, J. (2011). Effects of low level microwave radiation on carcinogenesis in Swiss Albino mice. Molecular and Cellular Biochemistry, 348, 191–197.
Paulraj, R., & Behari, J. (2012). Biochemical changes in rat brain exposed to low intensity 9.9 GHz microwave radiation. Cell Biochemistry and Biophysics, 63, 97–102. doi:10.1007/s12013-012-9344-3.
Nylund, R., & Leszczynski, D. (2004). Proteomics analysis of human endothelial cell line EA.hy926 after exposure to GSM 900 radiation. Proteomics, 4, 1359–1365.
Chauhan, V., Mariampillai, A., Bellier, P. V., Qutob, S. S., Gajda, G. B., Lemay, E., et al. (2006). Gene expression analysis of a human lymphoblastoma cell line exposed in vitro to an intermittent 1.9 GHz pulse-modulated radiofrequency field. Radiation Research, 165, 424–429.
Stagg, R. B., Hawel, L. H., Pastorian, K., Cain, C., Adey, W. R., & Byus, C. V. (2001). Effect of immobilization and concurrent exposure to a pulse-modulated microwave field on core body temperature, plasma ACTH and corticosteroid, and brain ornithine decarboxylase, Fos and Jun mRNA. Radiation Research, 155, 584–592.
Leszczynski, D., Joenvaara, S., Reivinen, J., & Kuokka, R. (2002). Non-thermal activation of the hsp27/p38MAPK stress pathway by mobile phone radiation in human endothelial cells molecular mechanism for cancer- and blood-brain barrier-related effects. Differentiation, 70, 120–129.
Lin, H., Opler, M., Head, M., Blank, M., & Goodman, R. (1997). Electromagnetic field exposure induces rapid, transitory heat shock factor activation in human cells. Journal of Cell Biochemistry, 66, 482–488.
Caraglia, M., Marra, M., Mancinelli, F., d’Ambrosio, G., Massa, R., Giordano, A., et al. (2005). Electromagnetic fields at mobile phone frequency induce apoptosis and inactivation of the multi-chaperone complex in human epidermoid cancer cells. Journal of` Cell Physiology, 204(2), 539–548.
French, P. W., Penny, R., Laurence, J. A., & McKenzie, D. R. (2001). Mobile phones, heat shock proteins and cancer. Differentiation, 67, 93–97.
Capri, M., Scarcella, E., Fumelli, C., Bianchi, E., Salvioli, S., Mesirca, P., et al. (2004). In vitro exposure of human lymphocytes to 900 MHz CW and GSM modulated radiofrequency: Studies of proliferation, apoptosis and mitochondrial membrane potential. Radiation Research, 162, 211–218.
Hook, G. J., Zhang, P., Lagroye, I., Li, L., Higashikubo, R., Moros, E. G., et al. (2004). Measurement of DNA damage and apoptosis in Molt-4 cells after in vitro exposure to radiofrequency radiation. Radiation Research, 16, 193–200.
Yoon, S., & Seger, R. (2006). The extracellular signal-regulated kinase: Multiple substrates regulate diverse cellular functions. Growth Factors, 24, 21–44.
Rubinfeld, H., & Seger, R. (2005). The ERK cascade: A prototype of MAPK signaling. Molecular Biotechnology, 31(2), 151–174.
Jin, M., Blank, M., & Goodman, R. (2000). ERK1/2 phosphorylation, induced by electromagnetic fields, diminishes during neoplastic transformation. Journal of Cellular Biochemistry, 78, 371–379.
Hayashi, I., Morishita, Y., Imai, K., Nakamura, M., Nakachi, K., & Hayashi, T. (2007). High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutation Research, 631, 55–61.
Kesari, K. K., Kumar, S., & Behari, J. (2011). Effects of radiofrequency electromagnetic waves exposure from cellular phone on reproductive pattern in male Wistar rats. Applied Biochemistry and Biotechnology, 164, 546–559.
Criswell, K. A., Krishna, G., Zielinski, D., Urda, G. A., Theiss, J. C., Juneau, P., et al. (1998). Use of acridine orange in: Flow cytometric assessment of micronuclei induction. Mutation Research, 414, 63–75.
Tian, Q., Streuli, M., Saito, H., Schlossman, S. F., & Paul, A. (1991). A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell, 67, 629–639.
Sambrook, J., Fritschi, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual. New York: Cold Spring Harbor Laboratory Press.
Meena, R., Kesari, K. K., & Paulraj, R. (2012). Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human breast cancer cells (MCF-7). Journal of Nanoparticle Research, 14(3), 712.
Xia, Z., Dickens, M., Raingeaud, J., Davis, R. J., & Greenberg, M. E. (1995). Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science, 270, 1326–1331.
Johnson, G. L., & Lapadat, R. (2002). Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science, 298, 1911–1912.
Pan, J., Xu, G., & Yeung, S. C. (2001). Cytochrome c release is upstream to activation of caspase-9, caspase-8, and caspase-3 in the enhanced apoptosis of anaplastic thyroid cancer cells induced by manumycin and paclitaxel. Journal of Clinical Endocrinology Metabolism, 86, 4731–4740.
Bossy-Wetzel, E., & Green, D. R. (1999). Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. Journal of Biological Chemistry, 274, 17484–17490.
D’Autréaux, B., & Toledano, M. B. (2007). ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nature Reviews Molecular Cell Biology, 8, 813–824.
Meena, R., Kumari, K., Kumar, J., Rajamani, P., Verma, H. N., & Kesari, K. K. (2013). Therapeutic approaches of melatonin in microwave radiations-induced oxidative stress-mediated toxicity on male fertility pattern of Wistar rats. Electromagnetic Biology and Medicine,. doi:10.3109/15368378.2013.781035.
Lai, H., & Singh, N. P. (1997). Melatonin and N-tert-butyl-α-phenylnitrone blocked 60-Hz magnetic field-induced DNA single and double strand breaks in rat brain cells. Journal Pineal Research, 22, 152–162.
Lai, H., & Singh, N. P. (1997). Melatonin and a spin-trap compound blocked radiofrequency radiation induced DNA strand breaks in rat brain cells. Bioelectromagnetics, 18, 446–454.
Lai, H., & Singh, N. P. (2004). Magnetic field-induced DNA strand breaks in brain cells of rat. Environmental Health Perspectives, 112(6), 87–694.
Paulraj, R., & Behari, J. (2006). Single strand DNA breaks in rat brain cells exposed to microwave radiation. Mutation Research, 596, 76–80.
Fumarola, C., & Guidotti, G. G. (2004). Stress-induced apoptosis: Toward a symmetry with receptor-mediated cell death. Apoptosis, 9, 77–82.
Yuan, Z. Q., Feldman, R. I., Sussman, G. E., Coppola, D., Nicosia, S. V., & Cheng, J. Q. (2003). AKT2 inhibition of Cisplatin-induced JNK/p38 and Bax activation by phosphorylation of ASK1: IMPLICATION OF AKT2 in chemoresistance. Journal of Biological Chemistry, 278, 23432–23440.
Davis, R. J. (2000). Signal transduction by the JNK group of MAP kinases. Cell, 103(2), 239–252.
Wada, T., & Penninger, J. M. (2004). Mitogen-activated protein kinases in apoptosis regulation. Oncogene, 23(16), 2838–2849.
Westwick, J. K., Bielawska, A. E., Dbaibo, G., Hannun, Y. A., & Brenner, D. A. (1995). Ceramide activates the stress-activated protein kinases. Journal Biological Chemistry, 270, 22689–22692.
Chen, Y. R., Meyer, C. F., & Tan, T. H. (1996). Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in gamma radiation-induced apoptosis. Journal of Biological Chemistry, 271, 631–634.
Cuvillier, O., Pirianov, G., Kleuser, B., Vanek, P. G., Coso, O. A., Gutkind, S., et al. (1996). Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature, 381, 800–803.
Zanke, B. W., Boudreau, K., Rubie, E., Winnett, E., Tibbles, L. A., Zon, L., et al. (1996). The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Current Biology, 6, 606–613.
Kurada, P., & White, K. (1998). Ras promotes cell survival in Drosophila by downregulating hid expression. Cell, 95, 319–329.
Choi, S. Y., Kim, M., Kang, C., Bae, G., Cho, C., Soh, J., et al. (2006). Activation of Bak and Bax through c-Abl-Protein Kinase C-p38 MAPK signaling in response to ionizing radiation in human non-small cell lung cancer cells. Journal of Biological Chemistry, 281(11), 7049–7059.
Cheng, A., Chan, S. L., Milhavet, O., Wang, S., & Mattson, M. P. (2001). p38 MAP kinase mediates nitric oxide induced apoptosis of neural progenitor cells. Journal of Biological Chemistry, 276, 43320–43327.
Choi, J. A., Park, M. T., Kang, C. M., Um, H. D., Bae, S., Lee, K. H., et al. (2004). Opposite effects of Ha-Ras and Ki-Ras on radiation-induced apoptosis via differential activation of PI3K/Akt and Rac/p38 mitogen-activated protein kinase signaling pathways. Oncogene, 23, 9–20.
Galan, A., Garcia-Bermejo, M. L., Troyano, A., Vilaboa, N. E., de Blas, E., Kazanietz, M. G., et al. (2000). Stimulation of p38 mitogen-activated protein kinase is an early regulatory event for the cadmium-induced apoptosis in human promonocytic cells. Journal of Biological Chemistry, 275, 11418–11424.
Acknowledgments
Authors are thankful to the Council for Scientific and Industrial Research [CSIR Project Ref. No. 37(1536)/12/EMR-II], New Delhi, for the financial assistance. Authors are also thankful to the reviewers of this paper for their important suggestions and corrections throughout the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kavindra Kumar Kesari and Ramovatar Meena have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Kesari, K.K., Meena, R., Nirala, J. et al. Effect of 3G Cell Phone Exposure with Computer Controlled 2-D Stepper Motor on Non-thermal Activation of the hsp27/p38MAPK Stress Pathway in Rat Brain. Cell Biochem Biophys 68, 347–358 (2014). https://doi.org/10.1007/s12013-013-9715-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9715-4