Abstract
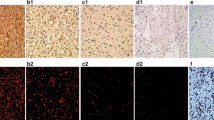
Ezrin is overexpressed in a variety of neoplastic cells and is involved in the later stages of tumor progression and metastasis. The present study investigated the expression and functional significance of ezrin in human brain astrocytoma. Ezrin expression was examined in specimens from healthy human brains (10 autopsies) or human astrocytoma (107 cases) by immunohistochemistry. All healthy specimens were negative for ezrin expression, while this expression was positive in a great majority of human astrocytoma tissues (96/107; 89.7 %; p < 0.05 vs. healthy). Ezrin expression was positively correlated with tumor grade (r = 0.551, p < 0.01). Analysis of clinicopathologic data revealed that the post-operation disease-free survival times were significantly (p < 0.001) different between those with a strong positive ezrin expression and those with a weak or negative expression. Specifically, median DFS in patients with a strongly positive ezrin expression was 13 months (range 2–46 months), while it was significantly (p < 0.001) longer in patients with weakly positive or negative expression (median of 28 months, range 6–56 months). In conclusion, there is a strong association between ezrin expression and increased malignancy in astrocytoma. Thus, enhanced ezrin expression may play an important role in the development of astrocytoma. Our results further indicate that ezrin may be useful for grading of astrocytoma and as a molecular marker for the prognosis.


Similar content being viewed by others
References
Schneider, T., Mawrin, C., Scherlach, C., Skalej, M., & Firsching, R. (2010). Gliomas in adults. Deutsches Aerzteblatt International, 107, 799–807.
Jiang, T., Tang, G. F., Lin, Y., Peng, X. X., Zhang, X., Zhai, X. W., et al. (2011). Prevalence estimates for primary brain tumors in China: A multi-center cross-sectional study. Chinese Medical Journal, 124, 2578–2583.
Wong, E. T., & Yamaguchi, N. H. (2011). Treatment advances for glioblastoma. Expert Review of Neurotherapeutics, 11, 1343–1345.
Grossman, S. A., Ye, X., Piantadosi, S., Desideri, S., Nabors, L. B., Rosenfeld, M., et al. (2010). Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clinical Cancer Research, 16, 2443–2449.
Ren, L., Hong, S. H., Cassavaugh, J., Osborne, T., Chou, A. J., Kim, S. Y., et al. (2009). The actin-cytoskeleton linker protein ezrin is regulated during osteosarcoma metastasis by PKC. Oncogene, 28, 792–802.
Kim, E. K., Park, J. M., Lim, S., Choi, J. W., Kim, H. S., Seok, H., et al. (2011). Activation of AMP-activated protein kinase is essential for lysophosphatidic acid-induced cell migration in ovarian cancer cells. Journal of Biological Chemistry, 286, 24036–24045.
Lorentzen, A., Bamber, J., Sadok, A., Elson-Schwab, I., & Marshall, C. J. (2011). An ezrin-rich, rigid uropod-like structure directs movement of amoeboid blebbing cells. Journal of Cell Science, 124, 1256–1267.
Hertweck, M. K., Erdfelder, F., & Kreuzer, K. A. (2011). CD44 in hematological neoplasias. Annals of Hematology, 90, 493–508.
Geiger, K. D., Stoldt, P., Schlote, W., & Derouiche, A. (2000). Ezrin immunoreactivity is associated with increasing malignancy of astrocytic tumors but is absent in oligodendrogliomas. American Journal of Pathology, 157, 1785–1793.
Tynninen, O., Carpen, O., Jaaskelainen, J., Paavonen, T., & Paetau, A. (2004). Ezrin expression in tissue microarray of primary and recurrent gliomas. Neuropathology and Applied Neurobiology, 30, 472–477.
Louis, D. N., Ohgaki, H., Wiestler, O. D., Cavenee, W. K., Burger, P. C., Jouvet, A., et al. (2007). The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathologica, 114, 97–109.
Mathew, J., Hines, J. E., Obafunwa, J. O., Burr, A. W., Toole, K., & Burt, A. D. (1996). CD44 is expressed in hepatocellular carcinomas showing vascular invasion. The Journal of Pathology, 179, 74–79.
Louvet-Vallee, S. (2000). ERM proteins: From cellular architecture to cell signaling. Biology of the Cell, 92, 305–316.
Bretscher, A., Edwards, K., & Fehon, R. G. (2002). ERM proteins and merlin: Integrators at the cell cortex. Nature Reviews Molecular Cell Biology, 3, 586–599.
Cassano, N., Loconsole, F., Amoruso, A., Coviello, C., Filieri, M., Filotico, R., et al. (2004). Infliximab monotherapy for refractory psoriasis: Preliminary results. International Journal of Immunopathology and Pharmacology, 17, 373–380.
Gronholm, M., Teesalu, T., Tyynela, J., Piltti, K., Bohling, T., Wartiovaara, K., et al. (2005). Characterization of the NF2 protein merlin and the ERM protein ezrin in human, rat, and mouse central nervous system. Molecular and Cellular Neuroscience, 28, 683–693.
Pang, S. T., Fang, X., Valdman, A., Norstedt, G., Pousette, A., Egevad, L., et al. (2004). Expression of ezrin in prostatic intraepithelial neoplasia. Urology, 63, 609–612.
Ohtani, K., Sakamoto, H., Rutherford, T., Chen, Z., Kikuchi, A., Yamamoto, T., et al. (2002). Ezrin, a membrane-cytoskeletal linking protein, is highly expressed in atypical endometrial hyperplasia and uterine endometrioid adenocarcinoma. Cancer Letters, 179, 79–86.
Khanna, C., Wan, X., Bose, S., Cassaday, R., Olomu, O., Mendoza, A., et al. (2004). The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nature Medicine, 10, 182–186.
Khanna, C., Khan, J., Nguyen, P., Prehn, J., Caylor, J., Yeung, C., et al. (2001). Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Research, 61, 3750–3759.
Chai, L., Sun, K., Guo, L., Zhang, H., & Lu, S. (2007). Expression of ezrin and CD44–v6 in human esophageal squamous cell carcinoma and its clinical significance. Chinese Journal of Clinical Oncology, 29, 685–688. In Chinese.
Elzagheid, A., Korkeila, E., Bendardaf, R., Buhmeida, A., Heikkila, S., Vaheri, A., et al. (2008). Intense cytoplasmic ezrin immunoreactivity predicts poor survival in colorectal cancer. Human Pathology, 39, 1737–1743.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mao, J., Yuan, Xr., Xu, Ss. et al. Expression and Functional Significance of Ezrin in Human Brain Astrocytoma. Cell Biochem Biophys 67, 1507–1511 (2013). https://doi.org/10.1007/s12013-013-9653-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9653-1