Abstract
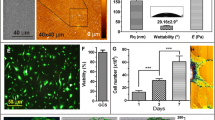
In the present study, we evaluated whether stem cell-to-tenocyte differentiation could be evaluated via measurement of the mechanical properties of the cell. We used mechanical uniaxial cyclic stretching to induce the differentiation of human bone marrow mesenchymal stem cells into tenocytes. The cells were subjected to cyclic elongation of 10 or 15 % at a cyclic frequency of 1 Hz for 24 or 48 h, and differentiation was assessed by real-time PCR (rtPCR) determination of messenger RNA expression levels for four commonly used markers of stem cell-to-tenocyte differentiation: type I collagen, type III collagen, tenascin-C, and scleraxis. The rtPCR results showed that cells subjected to 10 % cyclic elongation for 24 or 48 h differentiated into tenocytes. Atomic force microscopy (AFM) was then used to measure the force curves around the cell nuclei, and the AFM data were used to calculate the elastic moduli of the cell surfaces. The elastic modulus values of the control (non-stretched) cells differed significantly from those of cells stretched at 10 % for 24 or 48 h (P < 0.01). Confocal fluorescence microscopic observations of actin stress fibers suggested that the change in elastic modulus was ascribable to the development of the cellular cytoskeleton during the differentiation process. Therefore, we conclude that the atomic force microscopic measurement of the elastic modulus of the cell surface can be used to evaluate stem cell-to-tenocyte differentiation.






Similar content being viewed by others
References
Wolfman, N. M., Hattersley, G., Cox, K., Celeste, A. J., Nelson, R., Yamaji, N., et al. (1997). Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. Journal of Clinical Investigation, 100(2), 321–330.
Wang, J. H. C. (2006). Mechanobiology of tendon. Journal of Biomechanics, 39(9), 1563–1583.
Goh, J. C. H., Ouyang, H. W., Teoh, S. H., Chan, C. K. C., & Lee, E. H. (2003). Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Engineering, 9(1), S31–S44.
Sharma, P., & Maffulli, N. (2006). Biology of tendon injury: Healing, modeling and remodeling. Journal of Musculoskeletal and Neuronal Interactions, 6(2), 181–190.
Sharma, P., & Maffulli, N. (2005). Current concepts review tendon injury and tendinopathy: Healing and repair. Journal of Bone and Joint Surgery American Volume, 87A(1), 187–202.
Miyashita, H., Ochi, M., & Ikuta, Y. (1997). Histological and biomechanical observations of the rabbit patellar tendon after removal of its central one-third. Archives of Orthopaedic and Trauma Surgery, 116(8), 454–462.
Tohyama, H., Yasuda, K., Kitamura, Y., Yamamoto, E., & Hayashi, K. (2003). The changes in mechanical properties of regenerated and residual tissues in the patellar tendon after removal of its central portion. Clinical Biomechanics, 18(8), 765–772.
Chan, B. P., Fu, S. C., Qin, L., Rolf, C., & Chan, K. M. (1998). Pyridinoline in relation to ultimate stress of the patellar tendon during healing: An animal study. Journal of Orthopaedic Research, 16(5), 597–603.
Bagnaninchi, P. O., Yang, Y., El Haj, A. J., & Maffulli, N. (2007). Tissue engineering for tendon repair. British Journal of Sports Medicine, 41(8), e10.
Bullough, R., Finnigan, T., Kay, A., Maffulli, N., & Forsyth, N. R. (2008). Tendon repair through stem cell intervention: Cellular and molecular approaches. Disability and Rehabilitation, 30(20–22), 1746–1751.
Hampson, K., Forsyth, N. R., El Haj, A., & Maffulli, N. (2008). Tendon tissue engineering. In N. Ashammakhi, R. Reis & F. Chiellini (Eds.), Topics in tissue engineering, Chapter 3 (Vol. 4), Expertissues, E-book.
Pittenger, M. F., Mackay, A. M., Beck, S. C., Jaiswal, R. K., Douglas, R., Mosca, J. D., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–147.
Caplan, A., & Bruder, S. P. (2001). Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends in Molecular Medicine, 7(6), 259–264.
Giovannini, S., Brehm, W., Mainil-Varlet, P., & Nesic, D. (2008). Multilineage differentiation potential of equine blood-derived fibroblast-like cells. Differentiation, 76(2), 118–129.
Awad, H., Butler, D. L., Boivin, G. P., Smith, F. N. L., Malaviya, P., Huibregtse, B., et al. (1999). Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Engineering, 5(3), 267–277.
Friedens, A. J., Petrakov, K. V., Kuroleso, A. I., & Frolova, G. P. (1968). Heterotopic transplants of bone marrow—analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation, 6(2), 230–247.
Lee, C. H., Moioli, E. K., & Mao, J. J. (2006). Fibroblastic differentiation of human mesenchymal stem cells using connective tissue growth factor. Conference Proceedings of IEEE Engineering in Medicine and Biological Society, 1, 775–778.
Koch, H., Jadlowiec, J. A., Fu, F. H., Nonn, J., Merk, H. R., Hollinger, J. O., et al. (2004). The effect of growth/differentiation factor-5 (GDF-5) on genotype and phenotype in human adult mesenchymal stem cells. Zeitschrift für Orthopädie und ihre Grenzgebiete Deutsche Orthopädische Gesellschaft, 142(2), 248–253.
Park, A., Hogan, M. V., Kesturu, G. S., James, R., Balian, G., & Chhabra, A. B. (2010). Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Engineering, Part A, 16(9), 2941–2951.
Haddad-Weber, M., Prager, P., Kunz, M., Seefried, L., Jakob, F., Murray, M. M., et al. (2010). BMP12 and BMP13 gene transfer induce ligamentogenic differentiation in mesenchymal progenitor and anterior cruciate ligament cells. Cytotherapy, 12(4), 505–513.
Zeichen, J., van Griensven, M., & Bosch, U. (2000). The proliferative response of isolated human tendon fibroblasts to cyclic biaxial mechanical strain. American Journal of Sports Medicine, 28(6), 888–892.
Tanaka, H., Manske, P. R., Pruitt, D. L., & Larson, B. J. (1995). Effect of cyclic tension on lacerated flexor tendons in-vitro. Journal of Hand Surgery American Volume, 20A(3), 467–473.
Nöth, U., Schupp, K., Heymer, A., Kall, S., Jakob, F., Schutze, N., et al. (2005). Anterior cruciate ligament constructs fabricated from human mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy, 7(5), 447–455.
Yang, G. G., Crawford, R. C., & Wang, J. H. C. (2004). Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. Journal of Biomechanics, 37(10), 1543–1550.
Zhang, L., Tran, N., Chen, H. Q., Kahn, C. J. F., Marchal, S., Groubatch, F., et al. (2008). Time-related changes in expression of collagen types I and III and of tenascin-C in rat bone mesenchymal stem cells under co-culture with ligament fibroblasts or uniaxial stretching. Cell and Tissue Research, 332(1), 101–109.
Kuo, C. K., & Tuan, R. S. (2008). Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Engineering, Part A, 14(10), 1615–1627.
Altman, G. H., Horan, R. L., Martin, I., Farhadi, J., Stark, P. R. H., Volloch, V., et al. (2001). Cell differentiation by mechanical stress. FASEB Journal, 15(14), 270.
Farng, E., Urdaneta, A. R., Barba, D., Esmende, S., & McAllister, D. R. (2008). The effects of GDF-5 and uniaxial strain on mesenchymal stem cells in 3-D culture. Clinical Orthopaedics and Related Research, 466(8), 1930–1937.
Xu, B., Song, G., Ju, Y., Li, X., Song, Y., & Watanabe, S. (2012). RhoA/ROCK, cytoskeletal dynamics and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. Journal of Cell Physiology, 227(6), 2722–2729.
Mahaffy, R. E., Park, S., Gerde, E., Kas, J., & Shih, C. K. (2004). Quantitative analysis of the viscoelastic properties of thin regions of fibroblasts using atomic force microscopy. Biophysical Journal, 86(3), 1777–1793.
Takai, E., Costa, K. D., Shaheen, A., Hung, C. T., & Guo, X. E. (2005). Osteoblast elastic modulus measured by atomic force microscopy is substrate dependent. Annals of Biomedical Engineering, 33(7), 963–971.
Costa, K. D., Sim, A. J., & Yin, F. C. P. (2006). Non-Hertzian approach to analyzing mechanical properties of endothelial cells probed by atomic force microscopy. Journal of Biomechanical Engineering: The ASME, 128(2), 176–184.
Simon, A., Cohen-Bouhacina, T., Porte, M. C., Aime, J. P., Amedee, J., Bareille, R., et al. (2003). Characterization of dynamic cellular adhesion of osteoblasts using atomic force microscopy. Cytometry, Part A, 54A(1), 36–47.
Titushkin, I., & Cho, M. (2007). Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophysical Journal, 93(10), 3693–3702.
Mizutani, T., Haga, H., & Kawabata, K. (2004). Cellular stiffness response to external deformation: Tensional homeostasis in a single fibroblast. Cell Motility and the Cytoskeleton, 59(4), 242–248.
Goldmann, W. H., Galneder, R., Ludwig, M., Xu, W. M., Adamson, E. D., Wang, N., et al. (1998). Differences in elasticity of vinculin-deficient F9 cells measured by magnetometry and atomic force microscopy. Experimental Cell Research, 239(2), 235–242.
Haga, H., Sasaki, S., Kawabata, K., Ito, E., Ushiki, T., & Sambongi, T. (2000). Elasticity mapping of living fibroblasts by AFM and immunofluorescence observation of the cytoskeleton. Ultramicroscopy, 82(1–4), 253–258.
Eyre, D. R., Paz, M. A., & Gallop, P. M. (1984). Cross-linking in collagen and elastin. Annual Review of Biochemistry, 53, 717–748.
Lejard, V., Brideau, G., Blasis, F., Salingcarnboriboon, R., Wagner, G., Roehrl, M. H. A., et al. (2007). Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. Journal of Biological Chemistry, 282(24), 17665–17675.
Lapiere, C. M., Nusgens, B., & Pierard, G. E. (1977). Interaction between collagen type-1 and type-3 in conditioning bundles organization. Connective Tissue Research, 5(1), 21–29.
Elefteriou, F., Exposito, J. Y., Garrone, R., & Lethias, C. (2001). Binding of tenascin-X to decorin. FEBS Letters, 495(1–2), 44–47.
Sneddon, I. N. (1965). The relation between load and penetration in the axisymmetric Boussinesq problem for a punch of arbitrary profile. International Journal of Engineering Science, 3(1), 47–57.
Radmacher, M. (2002). Measuring the elastic properties of living cells by the atomic force microscope. Methods in Cell Biology, 68, 67–90.
Wang, H. C., Ip, W., Boissy, R., & Grood, E. S. (1995). Cell orientation response to cyclically deformed substrates: Experimental validation of a cell model. Journal of Biomechanics, 28(12), 1543–1552.
Naruse, K., Yamada, T., & Sokabe, M. (1998). Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. American Journal of Physiology: Heart and Circulatory Physiology, 274(5), H1532–H1538.
Neidlinger-Wilke, C., Grood, E. S., Wang, J. H. C., Brand, R. A., & Claes, L. (2001). Cell alignment is induced by cyclic changes in cell length: studies of cells grown in cyclically stretched substrates. Journal of Orthopaedic Research, 19(2), 286–293.
Zhang, L., Kahn, C. J. F., Chen, H. Q., Tran, N., & Wang, X. (2008). Effect of uniaxial stretching on rat bone mesenchymal stem cell: Orientation and expressions of collagen types I and III and tenascin-C. Cell Biology International, 32(3), 344–352.
Chen, Y. J., Huang, C. H., Lee, I. C., Lee, Y. T., Chen, M. H., & Young, T. H. (2008). Effects of cyclic mechanical stretching on the mRNA expression of tendon/ligament-related and osteoblast-specific genes in human mesenchymal stem cells. Connective Tissue Research, 49(1), 7–14.
Park, J. S., Chu, J. S. F., Cheng, C., Chen, F. Q., Chen, D., & Li, S. (2004). Differential effects of equiaxial and uniaxial strain on mesenchymal stem cells. Biotechnology and Bioengineering, 88(3), 359–368.
Costa, K. D., Hucker, W. J., & Yin, F. C. P. (2002). Buckling of actin stress fibers: A new wrinkle in the cytoskeletal tapestry. Cell Motility and the Cytoskeleton, 52(4), 266–274.
Xu, B., Song, G., & Ju, Y. (2011). Effect of focal adhesion kinase on the regulation of realignment and tenogenic differentiation of human mesenchymal stem cells by mechanical stretch. Connective Tissue Research, 52(5), 373–379.
Kannus, P., Jozsa, L., & Jarvinnen, M. (2000). Basic science of tendons. In W. J. Garrett, K. Speer, & D. Kirkendall (Eds.), Principles and practice of orthopaedic sports medicine (pp. 21–37). Philadelphia: Lippincott Williams and Wilkins.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morita, Y., Mukai, T., Ju, Y. et al. Evaluation of Stem Cell-to-Tenocyte Differentiation By Atomic Force Microscopy to Measure Cellular Elastic Moduli. Cell Biochem Biophys 66, 73–80 (2013). https://doi.org/10.1007/s12013-012-9455-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-012-9455-x