Abstract
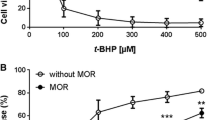
Although Akt is reported to play a role in morphine’s cardioprotection, little is known about the mechanism underlying morphine-induced Akt activation. This study aimed to define the molecular mechanism underlying morphine-induced Akt activation and to determine if the mechanism contributes to the protective effect of morphine on ischemia/reperfusion injury. In cardiac H9c2 cells, morphine increased Akt phosphorylation at Ser473, indicating that morphine upregulates Akt activity. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a major regulator of the phosphatidylinositol 3-kinase (PI3K)/Akt signaling, was not involved in the action of morphine on Akt activity. Morphine decreased the activity of PP2A, a major protein Ser/Thr phosphatase, and inhibition of PP2A with okadaic acid (OA) mimicked the effect of morphine on Akt activity. The effects of morphine on PP2A and Akt activities were inhibited by the reactive oxygen species (ROS) scavenger N-(2-mercaptopropionyl)glycine (MPG) and the mitochondrial KATP channel closer 5-hydroxydecanoate (5HD). In support, morphine could produce ROS and this was reversed by 5HD. Finally, the cardioprotective effect of morphine on ischemia–reperfusion injury was mimicked by OA but was suppressed by 5HD or MPG, indicating that protein phosphatases and ROS are involved in morphine’s protection. In conclusion, morphine upregulates Akt activity by inactivating protein Ser/Thr phosphatases via ROS, which may contribute to the cardioprotective effect of morphine.







Similar content being viewed by others
References
Gross, G. J. (2003). Role of opioids in acute and delayed preconditioning. Journal of Molecular and Cellular Cardiology, 35, 709–718.
Schultz, J. E., Hsu, A. K., & Gross, G. J. (1998). Ischemic preconditioning in the intact rat heart is mediated by delta1- but not mu- or kappa-opioid receptors. Circulation, 97, 1282–1289.
Schultz, J. J., Hsu, A. K., Nagase, H., & Gross, G. J. (1998). TAN-67, a delta-opioid receptor agonist, reduces infarct size via activation of Gi/o proteins and Katp channels. American Journal of Physiology, 274, H909–H914.
Huh, J., Gross, G. J., Nagase, H., & BT, L. (2001). Protection of cardiac myocytes via delta(1)-opioid receptors, protein kinase C, and mitochondrial K(ATP) channels. American Journal of Physiology Heart and Circulatory Physiology, 280, H377–H383.
Wu, S., Li, H.-Y., & Wong, T. M. (1999). Cardioprotection of preconditioning by metabolic inhibition in the rat ventricular myocyte. Circulation Research, 84, 1388–1395.
Wang, G. Y., Wu, S., Pei, J. M., Yu, X. C., & Wong, T. M. (2001). Kappa- but not delta-opioid receptors mediate effects of ischemic preconditioning on both infarct and arrhythmia in rats. American Journal of Physiology Heart and Circulatory Physiology, 280, H384–H391.
Miki, T., Cohen, M. V., & Downey, J. M. (1998). Opioid receptor contributes to ischemic preconditioning through protein kinase C activation in rabbits. Molecular and Cellular Biochemistry, 186, 3–12.
Fryer, R. M., Wang, Y., Hsu, A. K., & Gross, G. J. (2001). Essential activation of PKC-delta in opioid-initiated cardioprotection. American Journal of Physiology Heart and Circulatory Physiology, 280, H1346–H1353.
Liang, B. T., & Gross, G. J. (1999). Direct preconditioning of cardiac myocytes via opioid receptors and KATP channels. Circulation Research, 84, 1396–1400.
Fryer, R. M., Schultz, J. E. J., Hsu, A. K., & Gross, G. J. (1998). Pretreatment with tyrosine kinase inhibitors partially attenuates ischemic preconditioning in rat hearts. American Journal of Physiology, 275, H2009–H2015.
Fryer, R. M., Pratt, P. F., Hsu, A. K., & Gross, G. J. (2001). Differential activation of extracellular signal regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. Journal of Pharmacology and Experimental Therapeutics, 296, 642–649.
Fryer, R. M., Hsu, A. K., & Gross, G. J. (2001). ERK and p38 MAP kinase activation are components of opioid-induced delayed cardioprotection. Basic Research in Cardiology, 96, 136–142.
Gross, E. R., Hsu, A. K., & Gross, G. J. (2006). The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3beta. American Journal of Physiology, 291, H827–H834.
Peart, J. N., & Gross, G. J. (2006). Cardioprotective effects of acute and chronic opioid treatment are mediated via different signaling pathways. American Journal of Physiology, 291, H1746–H1753.
Gross, E. R., Hsu, A. K., & Gross, G. J. (2007). Diabetes abolishes morphine-induced cardioprotection via multiple pathways upstream of glycogen synthase kinase-3beta. Diabetes, 56, 127–136.
Cohen, M. V., Philipp, S., Krieg, T., Cui, L., Kuno, A., Solodushko, V., et al. (2007). Preconditioning-mimetics bradykinin and DADLE activate PI3-kinase through divergent pathways. Journal of Molecular and Cellular Cardiology, 42, 842–851.
Forster, K., Kuno, A., Solenkova, N., Felix, S. B., & Krieg, T. (2007). The {delta}-opioid receptor agonist DADLE at reperfusion protects the heart through activation of pro-survival kinases via EGF receptor transactivation. American Journal of Physiology, 293, H1604–H1608.
Gross, E. R., Hsu, A. K., & Gross, G. J. (2004). Opioid-induced cardioprotection occurs via glycogen synthase kinase β inhibition during reperfusion in intact rat hearts. Circulation Research, 94, 960–966.
Matsui, T., & Rosenzweig, A. (2005). Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. Journal of Molecular and Cellular Cardiology, 38, 63–71.
Brazil, D. P., & Hemmings, B. A. (2001). Ten years of protein kinase B signalling: a hard Akt to follow. Trends in Biochemical Sciences, 26, 657–664.
Miyamoto, S., Murphy, A., & Brown, J. (2009). Akt mediated mitochondrial protection in the heart: metabolic and survival pathways to the rescue. Journal of Bioenergetics and Biomembranes, 41, 169–180.
Mocanu, M. M., & Yellon, D. M. (2007). PTEN, the Achilles’ heel of myocardial ischaemia/reperfusion injury? British Journal of Pharmacology, 150, 833–838.
Millward, T. A., Zolnierowicz, S., & Hemmings, B. A. (1999). Regulation of protein kinase cascades by protein phosphatase 2A. Trends in Biochemical Sciences, 24, 186–191.
Lee, S.-R., Yang, K.-S., Kwon, J., Lee, C., Jeong, W., & Rhee, S. G. (2002). Reversible inactivation of the tumor suppressor PTEN by H2O2. The Journal of Biological Chemistry, 277, 20336–20342.
Hausenloy, D. J., & Yellon, D. M. (2006). Survival kinases in ischemic preconditioning and postconditioning. Cardiovascular Research, 70, 240–253.
Jang, Y. G., Xi, J. K., Wang, H. H., Mueller, R. A., Norfleet, E. A., & Xu, Z. L. (2008). Postconditioning prevents reperfusion injury by activating delta-opioid receptors. Anesthesiology, 108, 243–250.
Cai, Z., & Semenza, G. L. (2005). PTEN activity is modulated during ischemia and reperfusion: Involvement in the induction and decay of preconditioning. Circulation Research, 97, 1351–1359.
Mensah, K., Mocanu, M. M., & Yellon, D. M. (2005). Failure to protect the myocardium against ischemia/reperfusion injury after chronic atorvastatin treatment is recaptured by acute atorvastatin treatment: A potential role for phosphatase and tensin homolog deleted on chromosome ten? Journal of the American College of Cardiology, 45, 1287–1291.
Gericke, A., Munson, M., & Ross, A. H. (2006). Regulation of the PTEN phosphatase. Gene, 374, 1–9.
Cohen, M. V., Yang, X.-M., Liu, G. S., Heusch, G., & Downey, J. M. (2001). Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial K(ATP) channels. Circulation Research, 89, 273–278.
Vanhaesebroeck, B., & Alessi, D. R. (2000). The PI3K-PDK1 connection: more than just a road to PKB. Biochemical Journal, 346, 561–576.
Barthel, A., & Klotz, L. O. (2005). Phosphoinositide 3-kinase signaling in the cellular response to oxidative stress. Biological Chemistry, 386, 207–216.
Cohen, P. T. W. (1997). Novel protein serine/threonine phosphatases: Variety is the spice of life. Trends in Biochemical Sciences, 22, 245–251.
Weinbrenner, C., Baines, C. P., Liu, G.-S., Armstrong, S. C., Ganote, C. E., Walsh, A. H., et al. (1998). Fostriecin, an inhibitor of protein phosphatase 2A, limits myocardial infarct size even when administered after onset of ischemia. Circulation, 98, 899–905.
Armstrong, S. C., & Ganote, C. E. (1992). Effects of the protein phosphatase inhibitors okadaic acid and calyculin A on metabolically inhibited and ischaemic isolated myocytes. Journal of Molecular and Cellular Cardiology, 24, 869–884.
Lee, S., Chanoit, G., McIntosh, R., Zvara, D. A., & Xu, Z. L. (2009). Molecular mechanism underlying Akt activation in zinc-induced cardioprotection. American Journal of Physiology, 297, H569–H575.
Chen, L., Liu, L., & Huang, S. (2008). Cadmium activates the mitogen-activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radical Biology and Medicine, 45, 1035–1044.
Whisler, R. L., Goyette, M. A., Grants, I. S., & Newhouse, Y. G. (1995). Sublethal levels of oxidant stress stimulate multiple serine/threonine kinases and suppress protein phosphatases in Jurkat T cells. Archives of Biochemistry and Biophysics, 319, 23–35.
Downey, J., Davis, A., & Cohen, M. (2007). Signaling pathways in ischemic preconditioning. Heart Failure Reviews, 12, 181–188.
Fryer, R. M., Schultz, J. E. J., Hsu, A. K., & Gross, G. J. (1999). Importance of PKC and tyrosine kinase in single or multiple cycles of preconditioning in rat hearts. American Journal of Physiology, 276, H1229–H1235.
Yang, X., Cohen, M., & Downey, J. (2010). Mechanism of cardioprotection by early ischemic preconditioning. Cardiovascular Drugs and Therapy, 24, 225–234.
Yue, Y., Qin, Q., Cohen, M. V., Downey, J. M., & Critz, S. D. (2002). The relative order of mK(ATP) channels, free radicals and p38 MAPK in preconditioning’s protective pathway in rat heart. Cardiovascular Research, 55, 681–689.
Oldenburg, O., Qin, Q., Sharma, A., Cohen, M., Downey, J., & Benoit, J. (2002). Acetylcholine leads to free radical production dependent on K(ATP) channels, G(i) proteins, phosphatidylinositol 3-kinase and tyrosine kinase. Cardiovascular Research, 55, 544–552.
Oldenburg, O., Qin, Q., Krieg, T., Yang, X. M., Philipp, S., Critz, S. D., et al. (2003). Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mKATP channel opening and leads to cardioprotection. American Journal of Physiology Heart and Circulatory Physiology, 286, H468–H476.
McPherson, B. C., & Yao, Z. (2001). Morphine mimics preconditioning via free radical signals and mitochondrial KATP channels in myocytes. Circulation, 103, 290–295.
Acknowledgments
This study was supported by Grant 2007136 from Bureau of Education, Hebei Province, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jingman Xu, Wei Tian, and Xiaolong Ma contributed equally to this study.
Rights and permissions
About this article
Cite this article
Xu, J., Tian, W., Ma, X. et al. The Molecular Mechanism Underlying Morphine-Induced Akt Activation: Roles of Protein Phosphatases and Reactive Oxygen Species. Cell Biochem Biophys 61, 303–311 (2011). https://doi.org/10.1007/s12013-011-9213-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-011-9213-5