Abstract
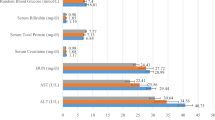
Arsenic contamination is a global health concern, primarily through contaminated groundwater and its entry into the food chain. The association between arsenic exposure and cardiovascular diseases (CVDs) is particularly alarming due to CVDs being the leading cause of death worldwide. Arsenic exposure has also been linked to changes in telomere length, mitochondrial DNA copy number (mtDNAcn), and deletion, further increasing the risk of CVDs. We aimed to determine whether arsenic exposure alters telomere length and mtDNAcn and deletion in a total of 50 CVD patients who underwent open heart surgery hailed from known arsenic-affected and unaffected areas in Bangladesh. Amount of arsenic was determined from the collected nails and cardiac tissues. Relative telomere length and mtDNAcn and deletion were quantified by qRT-PCR. The patients from arsenic-contaminated areas had higher average arsenic deposits in their fingers and toenails (P < 0.05) and higher cardiac tissue injury scores (P < 0.05). Moreover, approximately 1.5-fold shorter telomere length (P < 0.05, r = − 0.775), 1.2-fold decreased mtDNAcn (P < 0.05, r = − 0.797), and an 81-fold higher amount of mitochondrial DNA deletion (P < 0.05, r = 0.784) were observed in the patients who had higher arsenic deposition in their nails. Higher levels of arsenic exposure were found to be linked to shorter telomere length, decreased mtDNAcn, and increased mitochondrial DNA deletion in the patients from As-affected areas. It can also be anticipated that the correlation of arsenic exposure with telomere length, mtDNAcn, and deletion can be used as biomarkers for early diagnosis of arsenic-induced cardiovascular diseases.






Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this research work are available within the article and its supplementary materials. Any other data/information will also be available from the corresponding author on request.
References
Mandal, B. K., & Suzuki, K. T. (2002). Arsenic round the world: A review. Talanta, 58(1), 201–235.
Ahsan, H., Chen, Y., Liu, X., Siddique, A. B., Wu, T., Roy, S., Van Geen, K., Slavkovich, V., Levy, D., Factor-Litvak, P., Selhub, J., Gamble, M., Jorgensen, I., & Graziano, J. H. (2007). Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiology Biomarkers and Prevention, 16(6), 1270–1278. https://doi.org/10.1158/1055-9965.EPI-06-0676
Navas-Acien, A., Sharrett, A. R., Silbergeld, E. K., Schwartz, B. S., Nachman, K. E., Burke, T. A., & Guallar, E. (2005). Arsenic exposure and cardiovascular disease: A systematic review of the epidemiologic evidence. American Journal of Epidemiology, 162(11), 1037–1049. https://doi.org/10.1093/aje/kwi330
Xu, L., Mondal, D., & Polya, D. A. (2020). Positive association of cardiovascular disease (CVD) with chronic exposure to drinking water arsenic (As) at concentrations below the WHO provisional guideline value: A systematic review and meta-analysis. International Journal of Environmental Research and Public Health, 17(7), 2536. https://doi.org/10.3390/ijerph17072536
Chen, Y., Graziano, J. H., Parvez, F., Liu, M., Slavkovich, V., Kalra, T., Argos, M., Islam, T., Ahmed, A., Rakibuz-Zaman, M., Hasan, R., Sarwar, G., Levy, D., van Geen, A., Graziano, J. H., & Ahsan, H. (2013). A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environmental Health Perspectives, 121(7), 832–838. https://doi.org/10.1289/ehp.1205797
Rhyu, M. S. (1995). Telomeres, telomerase, and immortality. Journal of the National Cancer Institute, 87(12), 884–894. https://doi.org/10.1093/jnci/87.12.884
Saliques, S., Zeller, M., Lorin, J., Lorgis, L., Teyssier, J. R., Cottin, Y., & Rochette, L. (2010). Telomere length and cardiovascular disease. Archives of Cardiovascular Diseases, 103(8–9), 454–459. https://doi.org/10.1016/j.acvd.2010.08.002
Yeh, J. K., & Wang, C. Y. (2016). Telomeres and telomerase in cardiovascular diseases. Genes (Basel), 7(9), 58. https://doi.org/10.3390/genes7090058
Khaleda, L., Al-Forkan, M., Wali, F. B., Alam, M. J., Datta, A., Shawon, I. I., Hosain, N., & Rahman, M. Z. (2019). Effect of arsenic exposure on human telomerase reverse transcriptase (hTERT) gene expression: Risk of cardiovascular diseases: Arsenic exposure and cardiovascular diseases. Bangladesh Medical Research Council Bulletin, 45(1), 3–10. https://doi.org/10.3329/bmrcb.v45i1.41802
Bouffler, S. D., Blasco, M. A., Cox, R., & Smith, P. J. (2001). Telomeric sequences, radiation sensitivity and genomic instability. International Journal of Radiation Biology, 77(10), 995–1005. https://doi.org/10.1080/0955300011006932
Shay, J. W., Zou, Y., Hiyama, E., & Wright, W. E. (2001). Telomerase and cancer. Human molecular genetics, 10(7), 677–685. https://doi.org/10.1093/hmg/10.7.677
Gao, J., Roy, S., Tong, L., Argos, M., Jasmine, F., Rahaman, R., Rakibuz-Zaman, M., Parvez, F., Ahmed, A., Hore, S. K., Sarwar, G., Slavkovich, V., Yunus, M., Rahman, M., Baron, J. A., Graziano, J. H., Ahsan, H., & Pierce, B. L. (2015). Arsenic exposure, telomere length, and expression of telomere-related genes among Bangladeshi individuals. Environmental Research, 136, 462–469. https://doi.org/10.1016/j.envres.2014.09.040
Ameer, S. S., Xu, Y., Engström, K., Li, H., Tallving, P., Nermell, B., Boemo, A., Parada, L. A., Peñaloza, L. G., Concha, G., Harari, F., Vahter, M., & Broberg, K. (2016). Exposure to inorganic arsenic is associated with increased mitochondrial DNA copy number and longer telomere length in peripheral blood. Frontiers in Cell and Developmental Biology. https://doi.org/10.3389/fcell.2016.00087
Ferrario, D., Collotta, A., Carfi, M., Bowe, G., Vahter, M., Hartung, T., & Gribaldo, L. (2009). Arsenic induces telomerase expression and maintains telomere length in human cord blood cells. Toxicology, 260(1–3), 132–141. https://doi.org/10.1016/j.tox.2009.03.019
Zhang, T. C., Schmitt, M. T., & Mumford, J. L. (2003). Effects of arsenic on telomerase and telomeres in relation to cell proliferation and apoptosis in human keratinocytes and leukemia cells in vitro. Carcinogenesis, 24(11), 1811–1817. https://doi.org/10.1093/carcin/bgg141
Yue, P., Jing, S., Liu, L., Ma, F., Zhang, Y., Wang, C., Duan, H., Zhou, K., Hua, Y., Wu, G., & Li, Y. (2018). Association between mitochondrial DNA copy number and cardiovascular disease: Current evidence based on a systematic review and meta-analysis. PLoS One, 13(11), e0206003. https://doi.org/10.1371/journal.pone.0206003
Clay Montier, L. L., Deng, J. J., & Bai, Y. (2009). Number matters: Control of mammalian mitochondrial DNA copy number. Journal of Genetics and Genomics, 36(3), 125–131. https://doi.org/10.1016/S1673-8527(08)60099-5
Wallace, D. C. (2008). Mitochondria as Chi. Genetics, 179(2), 727–735. https://doi.org/10.1534/genetics.104.91769
Bayeva, M., Gheorghiade, M., & Ardehali, H. (2013). Mitochondria as a therapeutic target in heart failure. Journal of the American College of Cardiology, 61(6), 599–610. https://doi.org/10.1016/j.jacc.2012.08.1021
Fu, C., Chen, W., & Jin, Y. (2016). The complete mitochondrial genome of Phrynocephalus guinanensis (Reptilia, Squamata, Agamidae). Mitochondrial DNA, 27(2), 1103–1104. https://doi.org/10.3109/19401736.2014.933320
Taylor, R. W., & Turnbull, D. M. (2005). Mitochondrial DNA mutations in human disease. Nature Reviews Genetics, 6(5), 389–402. https://doi.org/10.1038/nrg1606
Campa, D., Barrdahl, M., Santoro, A., Severi, G., Baglietto, L., Omichessan, H., Tumino, R., Bueno-de-Mesquita, H. B., Peeters, P. H., Weiderpass, E., Chirlaque, M. D., Rodríguez-Barranco, M., Agudo, A., Gunter, M., Dossus, L., Krogh, V., Matullo, G., Trichopoulou, A., Travis, R. C., … Kaaks, R. (2018). Mitochondrial DNA copy number variation, leukocyte telomere length, and breast cancer risk in the European prospective investigation into cancer and nutrition (EPIC) study. Breast Cancer Research. https://doi.org/10.1186/s13058-018-0955-5
Phillips, N. R., Sprouse, M. L., & Roby, R. K. (2014). Simultaneous quantification of mitochondrial DNA copy number and deletion ratio: A multiplex real-time PCR assay. Scientific Reports. https://doi.org/10.1038/srep03887
Datta, A., Alam, M. J., Khaleda, L., & Al-Forkan, M. (2021). Protective effects of Corchorus olitorius and Butea monosperma against Arsenic induced aberrant methylation and mitochondrial DNA damage in Wistar rat model. Toxicology Reports, 8, 30–37. https://doi.org/10.1016/j.toxrep.2020.12.017
Zheng, J., Huang, T., Yu, Y., Hu, X., Yang, B., & Li, D. (2012). Fish consumption and CHD mortality: An updated meta-analysis of seventeen cohort studies. Public Health Nutrition, 15(4), 725–737. https://doi.org/10.1017/S1368980011002254
Al-Forkan, M., Wali, F. B., Khaleda, L., Alam, M. J., Chowdhury, R. H., Datta, A., Rahman, M. Z., Hosain, N., Maruf, M. F., Chowdhury, M. A. Q., Hasan, N. K. M. M., Shawon, I. I., & Raqib, R. (2021). Association of arsenic-induced cardiovascular disease susceptibility with genetic polymorphisms. Scientific Reports, 11(1), 6263. https://doi.org/10.1038/s41598-021-85780-8
Pourabdollah, M., Javadi, M., Shamaei, A., Ziazi, M., Dorudinia, L., Seyedmehdi, A., & Karimi, S. (2014). Qualification study of two genomic DNA extraction methods in different clinical samples. Tanaffos, 13(4), 41–47.
Gado, A. M., Adam, A. N., & Aldahmash, B. A. (2013). Cardiotoxicity induced by cyclophosphamide in rats: Protective effect of curcumin. Journal of Research in Environmental Science and Toxicology, 2(4), 87–95.
Cawthon, R. M. (2002). Telomere measurement by quantitative PCR. Nucleic Acids Research, 30(10), e47. https://doi.org/10.1093/nar/30.10.e47
Agahian, B., Lee, J. S., Nelson, J. H., & Johns, R. E. (1990). Arsenic levels in fingernails as a biological indicator of exposure to arsenic. American Industrial Hygiene Association Journal, 51(12), 646–651. https://doi.org/10.1080/15298669091370293
Cullen, W., & Reimer, K. J. (1989). Arsenic speciation in the environment. Chemical Reviews, 89, 713–764. https://doi.org/10.1021/cr00094a002
Wilhelm, M., Pesch, B., Wittsiepe, J., Jakubis, P., Miskovic, P., Keegan, T., Nieuwenhuijsen, M. J., & Ranft, U. (2005). Comparison of arsenic levels in fingernails with urinary As species as biomarkers of arsenic exposure in residents living close to a coal-burning power plant in Prievidza District, Slovakia. Journal of Exposure Analysis and Environmental Epidemiology, 15(1), 89–98. https://doi.org/10.1038/sj.jea.7500350
Al-Forkan, M., Islam, S., Akter, R., Alam, S. S., Khaleda, L., Rahman, Z., & Chowdhury, D. (2016). A sub-chronic exposure study of arsenic on hematological parameters, liver enzyme activities, histological studies and accumulation pattern of arsenic in organs of wistar albino rats. Journal of Cytology & Histology. https://doi.org/10.4172/2157-7099.1000S5:006
Rehman, K., Fatima, F., & Akash, M. S. H. (2019). Biochemical investigation of association of arsenic exposure with risk factors of diabetes mellitus in Pakistani population and its validation in animal model. Environmental Monitoring and Assessment. https://doi.org/10.1007/s10661-019-7670-2
Kumar, R., & Banerjee, T. K. (2016). Arsenic induced hematological and biochemical responses in nutritionally important catfish Clarias batrachus (L.). Toxicology Reports, 3, 148–152. https://doi.org/10.1016/j.toxrep.2016.01.001
Ola-Davies, O. E., & Akinrinde, A. S. (2016). Acute sodium Arsenite-induced hematological and biochemical changes in wistar rats: Protective effects of ethanol extract of Ageratum conyzoides. Pharmacognosy Research, 8, S26–S30. https://doi.org/10.4103/0974-8490.178645
Douglas, D., Samaha, R. J., & Barnes, A. (1975). Arsenic intoxication as a cause of megaloblastic anemia. Blood, 45(2), 241–246.
Hosen, S. M. I., Das, D., Kobi, R., Chowdhury, D. U. S., Alam, M. J., Rudra, B., Bakar, M. A., Islam, S., Rahman, Z., & Al-Forkan, M. (2016). Study of arsenic accumulation in rice and evaluation of protective effects of Chorchorus olitorius leaves against arsenic contaminated rice induced toxicities in Wistar albino rats. BMC Pharmacology and Toxicology. https://doi.org/10.1186/s40360-016-0091-8
Balakumar, P., & Kaur, J. (2009). Arsenic exposure and cardiovascular disorders: An overview. Cardiovascular Toxicology, 9(4), 169–176. https://doi.org/10.1007/s12012-009-9050-6
Samani, N. J., Boultby, R., Butler, R., Thompson, J. R., & Goodall, A. H. (2001). Telomere shortening in atherosclerosis. Lancet (London, England), 358(9280), 472–473. https://doi.org/10.1016/S0140-6736(01)05633-1
Liu, L., Trimarchi, J. R., Navarro, P., Blasco, M. A., & Keefe, D. L. (2003). Oxidative stress contributes to arsenic-induced telomere attrition, chromosome instability, and apoptosis. Journal of Biological Chemistry, 278(34), 31998–32004. https://doi.org/10.1074/jbc.M303553200
Pusceddu, I., Kleber, M., Delgado, G., Herrmann, W., März, W., & Herrmann, M. (2018). Telomere length and mortality in the ludwigshafen risk and cardiovascular health study. PLoS One, 13(6), e0198373. https://doi.org/10.1371/journal.pone.0198373
Hou, L., Zhu, Z. Z., Zhang, X., Nordio, F., Bonzini, M., Schwartz, J., Hoxha, M., Dioni, L., Marinelli, B., Pegoraro, V., Apostoli, P., Bertazzi, P. A., & Baccarelli, A. (2010). Airborne particulate matter and mitochondrial damage: A cross-sectional study. Environmental Health, 9(1), 48.
Liu, L. P., Cheng, K., Ning, M. A., Li, H. H., Wang, H. C., Li, F., Chen, S. Y., Qu, F. L., & Guo, W. Y. (2017). Association between peripheral blood cells mitochondrial DNA content and severity of coronary heart disease. Atherosclerosis, 261, 105–110. https://doi.org/10.1016/j.atherosclerosis.2017.02.013
Acknowledgements
The authors acknowledge the Cardiac Surgery Department and the Pathology Department of Chittagong Medical College and Hospital and the patients who enthusiastically participated in the research.
Funding
This research was carried out by self-funding.
Author information
Authors and Affiliations
Contributions
LK: Conceptualization, Methodology, Supervision, Review & Editing; SKB: Investigation, data analysis, Visualization & Data curation, Writing manuscript,; MARA: Formal analysis, Visualization, Data curation & manuscript preparation; RHC: Review & Editing manuscript, MJ: Methodology, Writing & Editing; AD:Writing Visualization & Data Curation, ZR: Methodology, Investigation & Review, NH: Sample Collection, Methodology & Review; MAF: Conceptualization, Methodology, Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interest.
Ethical Approval
Ethical clearance was taken from the Ethical Review Board (ERB) of Chittagong Medical College and Hospital, Chittagong. Each family was informed about the study and written Informed consent was obtained under a protocol accredited by the ethical review committee of Chittagong Medical College and Hospital.
Informed Consent
Written consent was obtained from all the parents with additional demographic data.
Additional information
Handling Editor: Lu Cai.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khaleda, L., Begum, S.K., Apu, M.A.R. et al. Arsenic-Induced Cardiovascular Diseases and their Correlation with Mitochondrial DNA Copy Number, Deletion, and Telomere Length in Bangladeshi Population. Cardiovasc Toxicol 24, 27–40 (2024). https://doi.org/10.1007/s12012-023-09812-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-023-09812-7