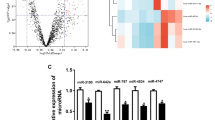
Abstract
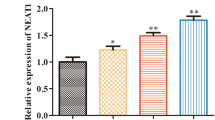
Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), a long non-coding RNA (lncRNA), has been confirmed to recruit enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) to regulate cardiomyocyte apoptosis in diabetic cardiomyopathy. However, whether the similar regulatory axis exists in sepsis-induced myocardial dysfunction (SIMD) has not been clearly established. The current study sought to define the mechanism governing MALAT1-mediated EZH2 in SIMD. MALAT1 was significantly upregulated in lipopolysaccharide-induced cardiomyocytes. Depletion of MALAT1 by caudal vein injection of small interfering RNA targeting MALAT1 alleviated myocardial injury in SIMD rats, restored cardiac function, reduced oxidative stress production and fibrosis, and inhibited inflammatory factors and apoptosis in myocardial tissues. Moreover, MALAT1 bound to EZH2 and promoted EZH2 activity in the nucleus of cardiomyocytes. EZH2 repressed ubiquitin-specific peptidase 22 (USP22) expression through H3K27me3 modification. EZH2 elevation aggravated the cardiac injury in SIMD rats, while USP22 upregulation inhibited the effect of EZH2, which reduced the cardiac injury in SIMD rats. Taken together, MALAT1 decreased USP22 expression by interacting with EZH2, thereby worsening SIMD, highlighting an attractive therapeutic strategy for SIMD.









Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Deutschman, C. S., & Tracey, K. J. (2014). Sepsis: Current dogma and new perspectives. Immunity, 40(4), 463–475. https://doi.org/10.1016/j.immuni.2014.04.001
Kumar, S., Tripathy, S., Jyoti, A., & Singh, S. G. (2019). Recent advances in biosensors for diagnosis and detection of sepsis: A comprehensive review. Biosensors & Bioelectronics, 124–125, 205–215. https://doi.org/10.1016/j.bios.2018.10.034
Hollenberg, S. M., & Singer, M. (2021). Pathophysiology of sepsis-induced cardiomyopathy. Nature Reviews Cardiology, 18(6), 424–434. https://doi.org/10.1038/s41569-020-00492-2
Walley, K. R. (2018). Sepsis-induced myocardial dysfunction. Current Opinion in Critical Care, 24(4), 292–299. https://doi.org/10.1097/MCC.0000000000000507
Lin, Y., Xu, Y., & Zhang, Z. (2020). Sepsis-Induced Myocardial Dysfunction (SIMD): The pathophysiological mechanisms and therapeutic strategies targeting mitochondria. Inflammation, 43(4), 1184–1200. https://doi.org/10.1007/s10753-020-01233-w
Boon, R. A., Jae, N., Holdt, L., & Dimmeler, S. (2016). Long noncoding RNAs: From clinical genetics to therapeutic targets? Journal of the American College of Cardiology, 67(10), 1214–1226. https://doi.org/10.1016/j.jacc.2015.12.051
Zhang, T. N., Goodwin, J. E., Liu, B., Li, D., Wen, R., Yang, N., Xia, J., Zhou, H., Zhang, T., Song, W. L., & Liu, C. F. (2019). Characterization of long noncoding RNA and mRNA profiles in sepsis-induced myocardial depression. Molecular Therapy—Nucleic Acids, 17, 852–866. https://doi.org/10.1016/j.omtn.2019.07.020
Hu, H., Wu, J., Yu, X., Zhou, J., Yu, H., & Ma, L. (2019). Long non-coding RNA MALAT1 enhances the apoptosis of cardiomyocytes through autophagy inhibition by regulating TSC2-mTOR signaling. Biological Research, 52(1), 58. https://doi.org/10.1186/s40659-019-0265-0
Wang, J., & Wang, G. G. (2020). No easy way out for EZH2: Its pleiotropic, noncanonical effects on gene regulation and cellular function. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms21249501
Shyni, G. L., Renjitha, J., Sasidhar Somappa, & Raghu, K. G. (2021). Zerumin A attenuates the inflammatory responses in LPS-stimulated H9c2 cardiomyoblasts. Journal of Biochemical and Molecular Toxicology, 35(6), 1–11. https://doi.org/10.1002/jbt.22777
Wu, B., Ni, H., Li, J., Zhuang, X., Zhang, J., Qi, Z., Chen, Q., Wen, Z., Shi, H., Luo, X., & Jin, B. (2017). The impact of circulating mitochondrial DNA on cardiomyocyte apoptosis and myocardial injury after TLR4 activation in experimental autoimmune myocarditis. Cellular Physiology and Biochemistry, 42(2), 713–728. https://doi.org/10.1159/000477889
Zhang, Y., Huang, H., Liu, W., Liu, S., Wang, X. Y., Diao, Z. L., Zhang, A. H., Guo, W., Han, X., Dong, X., & Katilov, O. (2021). Endothelial progenitor cells-derived exosomal microRNA-21-5p alleviates sepsis-induced acute kidney injury by inhibiting RUNX1 expression. Cell Death & Disease, 12(4), 335. https://doi.org/10.1038/s41419-021-03578-y
L’Heureux, M., Sternberg, M., Brath, L., Turlington, J., & Kashiouris, M. G. (2020). Sepsis-induced cardiomyopathy: A comprehensive review. Current Cardiology Reports, 22(5), 35. https://doi.org/10.1007/s11886-020-01277-2
Lv, X., & Wang, H. (2016). Pathophysiology of sepsis-induced myocardial dysfunction. Military Medical Research, 3, 30. https://doi.org/10.1186/s40779-016-0099-9
Li, C., Liu, Y., Qin, J., Liu, Y., Ma, L., Zhang, S., Wang, J., & Wang, S. (2021). Profiles of differentially expressed long noncoding RNAs and messenger RNAs in the myocardium of septic mice. Annals of Translational Medicine, 9(3), 199. https://doi.org/10.21037/atm-20-3830
Hu, H., Wu, J., Li, D., Zhou, J., Yu, H., & Ma, L. (2018). Knockdown of lncRNA MALAT1 attenuates acute myocardial infarction through miR-320-Pten axis. Biomedicine & Pharmacotherapy, 106, 738–746. https://doi.org/10.1016/j.biopha.2018.06.122
Cui, N., Liang, Y., Wang, J., Liu, B., Wei, B., & Zhao, Y. (2021). Minocycline attenuates oxidative and inflammatory injury in a intestinal perforation induced septic lung injury model via down-regulating lncRNA MALAT1 expression. International Immunopharmacology, 100, 108115. https://doi.org/10.1016/j.intimp.2021.108115
Jia, P., Wu, N., Jia, D., & Sun, Y. (2019). Downregulation of MALAT1 alleviates saturated fatty acid-induced myocardial inflammatory injury via the miR-26a/HMGB1/TLR4/NF-kappaB axis. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 12, 655–665. https://doi.org/10.2147/DMSO.S203151
Jing, H., Wang, C., Zhao, L., Cheng, J., Qin, P., & Lin, H. (2021). Propofol protects cardiomyocytes from hypoxia/reoxygenation injury via regulating MALAT1/miR-206/ATG3 axis. Journal of Biochemical and Molecular Toxicology, 35(10), e22880. https://doi.org/10.1002/jbt.22880
Liu, L., Yan, L. N., & Sui, Z. (2021). MicroRNA-150 affects endoplasmic reticulum stress via MALAT1-miR-150 axis-mediated NF-kappaB pathway in LPS-challenged HUVECs and septic mice. Life Sciences, 265, 118744. https://doi.org/10.1016/j.lfs.2020.118744
Han, L., & Yang, L. (2021). Multidimensional mechanistic spectrum of long non-coding RNAs in heart development and disease. Front Cardiovasc Med, 8, 728746. https://doi.org/10.3389/fcvm.2021.728746
Muthumani, M., & Prabu, S. M. (2014). Silibinin potentially attenuates arsenic-induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Cardiovascular Toxicology, 14(1), 83–97. https://doi.org/10.1007/s12012-013-9227-x
Qu, D., Sun, W. W., Li, L., Ma, L., Sun, L., Jin, X., Li, T., Hou, W., & Wang, J. H. (2019). Long noncoding RNA MALAT1 releases epigenetic silencing of HIV-1 replication by displacing the polycomb repressive complex 2 from binding to the LTR promoter. Nucleic Acids Research, 47(6), 3013–3027. https://doi.org/10.1093/nar/gkz117
Wang, C., Liu, G., Yang, H., Guo, S., Wang, H., Dong, Z., Li, X., Bai, Y., & Cheng, Y. (2021). MALAT1-mediated recruitment of the histone methyltransferase EZH2 to the microRNA-22 promoter leads to cardiomyocyte apoptosis in diabetic cardiomyopathy. Science of the Total Environment, 766, 142191. https://doi.org/10.1016/j.scitotenv.2020.142191
Yong, H., Wu, G., Chen, J., Liu, X., Bai, Y., Tang, N., Liu, L., & Wei, J. (2020). lncRNA MALAT1 accelerates skeletal muscle cell apoptosis and inflammatory response in sepsis by decreasing BRCA1 expression by recruiting EZH2. Mol Ther Nucleic Acids, 21, 1120–1121. https://doi.org/10.1016/j.omtn.2020.07.033
Yu, Z., Rayile, A., Zhang, X., Li, Y., & Zhao, Q. (2017). Ulinastatin protects against lipopolysaccharide-induced cardiac microvascular endothelial cell dysfunction via downregulation of lncRNA MALAT1 and EZH2 in sepsis. International Journal of Molecular Medicine, 39(5), 1269–1276. https://doi.org/10.3892/ijmm.2017.2920
Cai, L. J., Tu, L., Huang, X. M., Huang, J., Qiu, N., Xie, G. H., Liao, J. X., Du, W., Zhang, Y. Y., & Tian, J. Y. (2020). LncRNA MALAT1 facilitates inflammasome activation via epigenetic suppression of Nrf2 in Parkinson’s disease. Molecular Brain, 13(1), 130. https://doi.org/10.1186/s13041-020-00656-8
Liang, H., Huang, Q., Liao, M. J., Xu, F., Zhang, T., He, J., Zhang, L., & Liu, H. Z. (2019). EZH2 plays a crucial role in ischemia/reperfusion-induced acute kidney injury by regulating p38 signaling. Inflammation Research, 68(4), 325–336. https://doi.org/10.1007/s00011-019-01221-3
Liu, H., Chen, Z., Weng, X., Chen, H., Du, Y., Diao, C., Liu, X., & Wang, L. (2020). Enhancer of zeste homolog 2 modulates oxidative stress-mediated pyroptosis in vitro and in a mouse kidney ischemia-reperfusion injury model. The FASEB Journal, 34(1), 835–852. https://doi.org/10.1096/fj.201901816R
Peng, Y., Zhao, J. L., Peng, Z. Y., Xu, W. F., & Yu, G. L. (2020). Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death & Disease, 11(5), 317. https://doi.org/10.1038/s41419-020-2545-6
Melo-Cardenas, J., Zhang, Y., Zhang, D. D., & Fang, D. (2016). Ubiquitin-specific peptidase 22 functions and its involvement in disease. Oncotarget, 7(28), 44848–44856. https://doi.org/10.18632/oncotarget.8602
Ma, S., Sun, L., Wu, W., Wu, J., Sun, Z., & Ren, J. (2020). USP22 protects against myocardial ischemia-reperfusion injury via the SIRT1-p53/SLC7A11-dependent inhibition of ferroptosis-induced cardiomyocyte death. Frontiers in Physiology, 11, 551318. https://doi.org/10.3389/fphys.2020.551318
Funding
None.
Author information
Authors and Affiliations
Contributions
HX: conception and design of the research, drafted manuscript and performed main experiment; WY: contribution to validation and analyzed data; BCS: contribution to visualization, prepared figures and interpreted results of experiments. All authors reviewed and approved the final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no competing interests in this study.
Ethical Approval
All animal experiment protocols were approved by the Animal Care Committee of the Shandong Provincial Third Hospital in compliance with the guidelines proposed by the Guide for the Care and Use of Laboratory Animals.
Consent to Participate
All authors reviewed and approved the final version of manuscript.
Additional information
Handling Editor: Y. James Kang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, H., Ye, W. & Shi, B. LncRNA MALAT1 Regulates USP22 Expression Through EZH2-Mediated H3K27me3 Modification to Accentuate Sepsis-Induced Myocardial Dysfunction. Cardiovasc Toxicol 22, 813–830 (2022). https://doi.org/10.1007/s12012-022-09758-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-022-09758-2