Abstract
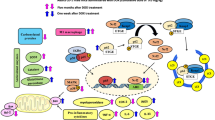
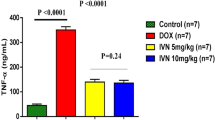
Doxorubicin (DOX) is one of the most widely used chemotherapeutic drugs, but its cardiotoxicity has been shown to be a dose-restricting factor during therapy. Finding new agents for reducing these complications is still in critical need. The current study aimed to evaluate the possible cardioprotective effect of hemin (HEM) in DOX-induced cardiotoxicity and exploring the role of toll like receptor-5/nuclear factor kappa-B/tumor necrosis factor-alpha (TLR-5/NF-κB/TNF-α) and nuclear factor erythroid 2-related factor-2/hemeoxygenase-1 (Nrf-2/HO-1) signaling pathways in mediating such effect. Wistar albino rats were randomly divided into five groups. They were administered DOX by interaperitoneal (i.p.) injection (15 mg/kg) on the 5th day of the experiment with or without HEM in different doses (2.5, 5, 10 mg/kg/day) i.p. for 7 days. Results showed that the DOX group had cardiotoxicity as manifested by a significant increase in cardiac enzymes, malondialdehyde (MDA), TLR-5, NF-κB, TNF-α, and cleaved caspase-3 levels with toxic histopathological changes. Based on these findings, HEM succeeded in reducing DOX-induced cardiotoxicity in a dose-dependent effect by stimulation of Nrf-2/HO-1 and inhibition of TLR-5/NF-κB/TNF-α pathways with subsequent antioxidant, anti-inflammatory, and anti-apoptotic effects.





Similar content being viewed by others
Data Availability
All data are available as supplementary material.
Code Availability
Not applicable.
References
Henninger, C., & Fritz, G. (2017). Statins in anthracycline-induced cardiotoxicity: Rac and Rho, and the heartbreakers. Cell Death & Disease, 8(1), e2564–e2564. https://doi.org/10.1038/cddis.2016.418
El-Zayat, S. R., Sibaii, H., & Mannaa, F. A. (2019). Toll-like receptors activation, signaling, and targeting: An overview. Bulletin of the National Research Centre, 43(1), 187. https://doi.org/10.1186/s42269-019-0227-2
Arslan, F., Smeets, M. B., O’Neill, L. A., Keogh, B., McGuirk, P., Timmers, L., Tersteeg, C., Hoefer, I. E., Doevendans, P. A., Pasterkamp, G., & de Kleijn, D. P. (2010). Myocardial ischemia/reperfusion injury is mediated by leukocytic toll-like receptor-2 and reduced by systemic administration of a novel anti-toll-like receptor-2 antibody. Circulation, 121(1), 80–90. https://doi.org/10.1161/circulationaha.109.880187
Yu, L., & Feng, Z. (2018). The role of toll-like receptor signaling in the progression of heart failure. Mediators of inflammation, 2018, 9874109–9874109. https://doi.org/10.1155/2018/9874109
Ehrentraut, H., Weber, C., Ehrentraut, S., Schwederski, M., Boehm, O., Knuefermann, P., Meyer, R., & Baumgarten, G. (2011). The toll-like receptor 4-antagonist eritoran reduces murine cardiac hypertrophy. European Journal of Heart Failure, 13(6), 602–610. https://doi.org/10.1093/eurjhf/hfr035
Cristofaro, P., & Opal, S. M. (2003). The toll-like receptors and their role in septic shock. Expert Opinion on Therapeutic Targets, 7(5), 603–612. https://doi.org/10.1517/14728222.7.5.603
Octavia, Y., Tocchetti, C. G., Gabrielson, K. L., Janssens, S., Crijns, H. J., & Moens, A. L. (2012). Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. Journal of Molecular and Cellular Cardiology, 52(6), 1213–1225. https://doi.org/10.1016/j.yjmcc.2012.03.006
Zhang, Y. W., Shi, J., Li, Y. J., & Wei, L. (2009). Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Archivum immunolgiae et therapiae experimentalis, 57(6), 435–445. https://doi.org/10.1007/s00005-009-0051-8
Ma, Q. (2013). Role of nrf2 in oxidative stress and toxicity. Annual Review of Pharmacology and Toxicology, 53, 401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320
Shan, H., Li, T., Zhang, L., Yang, R., Li, Y., Zhang, M., Dong, Y., Zhou, Y., Xu, C., Yang, B., Liang, H., Gao, X., & Shan, H. (2019). Heme oxygenase-1 prevents heart against myocardial infarction by attenuating ischemic injury-induced cardiomyocytes senescence. eBioMedicine, 39, 59–68. https://doi.org/10.1016/j.ebiom.2018.11.056
Refaie, M. M. M., El-Hussieny, M., Bayoumi, A. M. A., & Shehata, S. (2019). Mechanisms mediating the cardioprotective effect of carvedilol in cadmium induced cardiotoxicity. Role of eNOS and HO1/Nrf2 pathway. Environmental Toxicology and Pharmacology, 70, 103198. https://doi.org/10.1016/j.etap.2019.103198
Kelleni, M. T., Amin, E. F., & Abdelrahman, A. M. (2015). Effect of metformin and sitagliptin on doxorubicin-induced cardiotoxicity in rats: impact of oxidative stress, inflammation, and apoptosis. Journal of Toxicology, 2015, 424813. https://doi.org/10.1155/2015/424813
Paliwal, Y. K., Mehan, S., Bijjem, K. R., & Sharma, P. L. (2014). Renoprotective effect of ace inhibitor-lisinopril and heme oxygenase-1 inducer-hemin combination against streptozotocin induced advanced diabetic nephropathy in rats. Pharmacologia, 5, 60–75.
Buege, J. A., & Aust, S. D. (1978). Microsomal lipid peroxidation. In S. Fleischer & L. Packer (Eds.), Methods in enzymology (Vol. 52, pp. 302–310). Academic Press.
Moron, M. S., Depierre, J. W., & Mannervik, B. (1979). Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochimica et Biophysica Acta, 582(1), 67–78. https://doi.org/10.1016/0304-4165(79)90289-7
Abdel-Gaber, S. A., Geddawy, A., & Moussa, R. A. (2019). The hepatoprotective effect of sitagliptin against hepatic ischemia reperfusion-induced injury in rats involves Nrf-2/HO-1 pathway. Pharmacological Reports, 71(6), 1044–1049. https://doi.org/10.1016/j.pharep.2019.06.006
VanGuilder, H. D., Vrana, K. E., & Freeman, W. M. (2008). Twenty-five years of quantitative PCR for gene expression analysis. BioTechniques, 44(5), 619–626. https://doi.org/10.2144/000112776
Ewees, M. G., Messiha, B. A. S., Abdel-Bakky, M. S., Bayoumi, A. M. A., & Abo-Saif, A. A. (2019). Tempol, a superoxide dismutase mimetic agent, reduces cisplatin-induced nephrotoxicity in rats. Drug and Chemical Toxicology, 42(6), 657–664. https://doi.org/10.1080/01480545.2018.1485688
El-Agamy, D. S., Abo-Haded, H. M., & Elkablawy, M. A. (2016). Cardioprotective effects of sitagliptin against doxorubicin-induced cardiotoxicity in rats. Experimental Biology and Medicine (Maywood, N.J.), 241(14), 1577–1587. https://doi.org/10.1177/1535370216643418
Singal, P. K., & Iliskovic, N. (1998). Doxorubicin-induced cardiomyopathy. New England Journal of Medicine, 339(13), 900–905. https://doi.org/10.1056/nejm199809243391307
Ichikawa, Y., Ghanefar, M., Bayeva, M., Wu, R., Khechaduri, A., Naga Prasad, S. V., Mutharasan, R. K., Naik, T. J., & Ardehali, H. (2014). Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. The Journal of Clinical Investigation, 124(2), 617–630. https://doi.org/10.1172/jci72931
Zhang, Q.-L., Yang, J.-J., & Zhang, H.-S. (2019). Carvedilol (CAR) combined with carnosic acid (CAA) attenuates doxorubicin-induced cardiotoxicity by suppressing excessive oxidative stress, inflammation, apoptosis and autophagy. Biomedicine & Pharmacotherapy, 109, 71–83. https://doi.org/10.1016/j.biopha.2018.07.037
Adıyaman, M. Ş, Adıyaman, Ö. A., Dağlı, A. F., Karahan, M. Z., Kaya, İ, & Dağlı, M. N. (2020). Effects of grapeseed extract on doxorubicin-induced cardiotoxicity in rats. Herz. https://doi.org/10.1007/s00059-019-04888-w
Refaie, M. M. M., Shehata, S., El-Hussieny, M., Abdelraheem, W. M., & Bayoumi, A. M. A. (2020). Role of ATP-sensitive potassium channel (K(ATP)) and eNOS in mediating the protective effect of nicorandil in cyclophosphamide-induced cardiotoxicity. Cardiovascular Toxicology, 20(1), 71–81. https://doi.org/10.1007/s12012-019-09535-8
Ayala, A., Muñoz, M. F., & Argüelles, S. (2014). Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity, 2014, 360438. https://doi.org/10.1155/2014/360438
Zare, M. F. R., Rakhshan, K., Aboutaleb, N., Nikbakht, F., Naderi, N., Bakhshesh, M., & Azizi, Y. (2019). Apigenin attenuates doxorubicin induced cardiotoxicity via reducing oxidative stress and apoptosis in male rats. Life Sciences, 232, 116623. https://doi.org/10.1016/j.lfs.2019.116623
HAS, A. L., Alotaibi, M. F., Bin-Jumah, M., Elgebaly, H., & Mahmoud, A. M. (2019). Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomedicine & Pharmacotherapy, 111, 676–685. https://doi.org/10.1016/j.biopha.2018.12.112
Osburn, W. O., Wakabayashi, N., Misra, V., Nilles, T., Biswal, S., Trush, M. A., & Kensler, T. W. (2006). Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Archives of Biochemistry and Biophysics, 454, 7–15.
Saha, S., Buttari, B., Panieri, E., Profumo, E., & Saso, L. (2020). An overview of Nrf2 signaling pathway and its role in inflammation. Molecules, 25, 4574.
Bozoglu, T., Hinkel, R., & Kupatt, C. (2016). Therapeutic potential of heme oxygenase 1 in ischemia reperfusion injury. Journal of Transplantation Technologies, 6(3), 1000162.
Zhou, H., Liu, H., Porvasnik, S. L., Terada, N., Agarwal, A., Cheng, Y., & Visner, G. A. (2006). Heme oxygenase-1 mediates the protective effects of rapamycin in monocrotaline-induced pulmonary hypertension. Laboratory Investigation, 86(1), 62–71. https://doi.org/10.1038/labinvest.3700361
Botros, F. T., Schwartzman, M. L., Stier, C. T., Goodman, A. I., & Abraham, N. G. (2005). Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney International, 68(6), 2745–2755. https://doi.org/10.1111/j.1523-1755.2005.00745.x
Wang, R., Shamloul, R., Wang, X., Meng, Q., & Wu, L. (2006). Sustained normalization of high blood pressure in spontaneously hypertensive rats by implanted hemin pump. Hypertension, 48(4), 685–692. https://doi.org/10.1161/01.HYP.0000239673.80332.2f
Lakkisto, P., Kytö, V., Forsten, H., Siren, J. M., Segersvärd, H., Voipio-Pulkki, L. M., Laine, M., Pulkki, K., & Tikkanen, I. (2010). Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1alpha, SDF-1alpha and VEGF-B. European Journal of Pharmacology, 635(1–3), 156–164. https://doi.org/10.1016/j.ejphar.2010.02.050
Sherif, I. O. (2018). The effect of natural antioxidants in cyclophosphamide-induced hepatotoxicity: Role of Nrf2/HO-1 pathway. International Immunopharmacology, 61, 29–36. https://doi.org/10.1016/j.intimp.2018.05.007
Mansour, D. F., Saleh, D. O., & Mostafa, R. E. (2017). Genistein ameliorates cyclophosphamide—induced hepatotoxicity by modulation of oxidative stress and inflammatory mediators. Open Access Macedonian Journal of Medical Sciences, 5(7), 836–843. https://doi.org/10.3889/oamjms.2017.093
Elshater, A. A., Haridy, M. A. M., Salman, M. M. A., Fayyad, A. S., & Hammad, S. (2018). Fullerene C60 nanoparticles ameliorated cyclophosphamide-induced acute hepatotoxicity in rats. Biomedicine & Pharmacotherapy, 97, 53–59. https://doi.org/10.1016/j.biopha.2017.10.134
Yet, S. F., Perrella, M. A., Layne, M. D., Hsieh, C. M., Maemura, K., Kobzik, L., Wiesel, P., Christou, H., Kourembanas, S., & Lee, M. E. (1999). Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. The Journal of Clinical Investigation, 103(8), R23-29. https://doi.org/10.1172/jci6163
Chi, X., Guo, N., Yao, W., Jin, Y., Gao, W., Cai, J., & Hei, Z. (2016). Induction of heme oxygenase-1 by hemin protects lung against orthotopic autologous liver transplantation-induced acute lung injury in rats. Journal of Translational Medicine, 14(1), 35. https://doi.org/10.1186/s12967-016-0793-0
Martín, P. L., Ceccatto, P., Razori, M. V., Francés, D. E. A., Arriaga, S. M. M., Pisani, G. B., Martínez, A. I., Sánchez Pozzi, E. J., Roma, M. G., & Basiglio, C. L. (2019). Heme oxygenase-1 induction by hemin prevents oxidative stress-induced acute cholestasis in the rat. Clinical Science, 133(1), 117–134. https://doi.org/10.1042/CS20180675
Caballero, I., Boyd, J., Almiñana, C., Sánchez-López, J. A., Basatvat, S., Montazeri, M., Maslehat Lay, N., Elliott, S., Spiller, D. G., White, M. R. H., & Fazeli, A. (2017). Understanding the dynamics of toll-like receptor 5 response to flagellin and its regulation by estradiol. Scientific Reports, 7(1), 40981. https://doi.org/10.1038/srep40981
Xiao, B., Hong, L., Cai, X., Mei, S., Zhang, P., & Shao, L. (2019). The true colors of autophagy in doxorubicin-induced cardiotoxicity. Oncology Letters, 18(3), 2165–2172. https://doi.org/10.3892/ol.2019.10576
Ma, Z. G., Kong, C. Y., Wu, H. M., Song, P., Zhang, X., Yuan, Y. P., Deng, W., & Tang, Q. Z. (2020). Toll-like receptor 5 deficiency diminishes doxorubicin-induced acute cardiotoxicity in mice. Theranostics, 10(24), 11013–11025. https://doi.org/10.7150/thno.47516
Sandamali, J. A. N., Hewawasam, R. P., Jayatilaka, K., & Mudduwa, L. K. B. (2020). Cardioprotective potential of Murraya koenigii (L.) spreng. Leaf extract against doxorubicin-induced cardiotoxicity in rats. Evidence-Based Complementary and Alternative Medicine, 2020, 6023737. https://doi.org/10.1155/2020/6023737
Wei, J., Fan, G., Zhao, H., & Li, J. (2015). Heme oxygenase-1 attenuates inflammation and oxidative damage in a rat model of smoke-induced emphysema. International Journal of Molecular Medicine, 36(5), 1384–1392. https://doi.org/10.3892/ijmm.2015.2353
Collino, M., Pini, A., Mugelli, N., Mastroianni, R., Bani, D., Fantozzi, R., Papucci, L., Fazi, M., & Masini, E. (2013). Beneficial effect of prolonged heme oxygenase 1 activation in a rat model of chronic heart failure. Disease Models & Mechanisms, 6(4), 1012–1020. https://doi.org/10.1242/dmm.011528
Hu, J., Wu, Q., Wang, Z., Hong, J., Chen, R., Li, B., Hu, Z., Hu, X., & Zhang, M. (2019). Inhibition of CACNA1H attenuates doxorubicin-induced acute cardiotoxicity by affecting endoplasmic reticulum stress. Biomedicine & Pharmacotherapy, 120, 109475. https://doi.org/10.1016/j.biopha.2019.109475
Elmore, S. (2007). Apoptosis: A review of programmed cell death. Toxicologic pathology, 35(4), 495–516. https://doi.org/10.1080/01926230701320337
Jasim, S. T., Al-Kuraishy, H. M., & Al-Gareeb, A. I. (2019). Gingko biloba protects cardiomyocytes against acute doxorubicin induced cardiotoxicity by suppressing oxidative stress. Journal of Pakistan Medical Association, 69(38), 103–107.
Yang, F., Zhang, Y., Tang, Z., Shan, Y., Wu, X., & Liu, H. (2020). Hemin treatment protects neonatal rats from sevoflurane-induced neurotoxicity via the phosphoinositide 3-kinase/Akt pathway. Life Sciences, 242, 117151. https://doi.org/10.1016/j.lfs.2019.117151
Biswas, S. K. (2016). Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Medicine and Cellular Longevity, 2016, 5698931. https://doi.org/10.1155/2016/5698931
Acknowledgements
We'd like to thank Dr. Wedad M. Abdelraheem, Department of Medical Microbiology and Immunology, Faculty of Medicine, Minia University, El-Minia, Egypt for her generous help in rt-PCR part.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Dr. MMMR, Dr. SAA-G, and Dr. SS selected the point, performed the experimental part, wrote the manuscript, and sent it for publication. Dr. RAI performed and wrote the histopathology, immunohistochemistry, and revised the manuscript. Dr. AMAB performed and wrote the western blotting part and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Animal handling, medications, and animal sacrifice were carried out following the guidelines for the care of experimental animals and approved by the Institutional Ethical Committee, Faculty of Medicine, Minia University, Egypt according to the NIH Guide for taking care and use of laboratory animals. Approval No. 20:3/2021.
Informed Consent
Not applicable.
Research Involving Human Participants and/or Animals
Animals.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Handling Editor: Samuel S. W. Tay.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Refaie, M.M.M., Shehata, S., Ibrahim, R.A. et al. Dose-Dependent Cardioprotective Effect of Hemin in Doxorubicin-Induced Cardiotoxicity Via Nrf-2/HO-1 and TLR-5/NF-κB/TNF-α Signaling Pathways. Cardiovasc Toxicol 21, 1033–1044 (2021). https://doi.org/10.1007/s12012-021-09694-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09694-7