Abstract
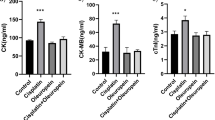
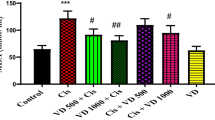
The cardiotoxicity of chemotherapeutic drugs as cisplatin has become a major issue in recent years. The present study investigates the efficacy of curcumin nanoparticles against the cardiotoxic effects of cisplatin by assessment of oxidative stress parameters, Na+,K+-ATPase, acetylcholinesterase (AchE) and tumor necrosis factor-alpha (TNF-α) in cardiac tissue in addition to serum lactate dehydrogenase (LDH). Rats were divided into three groups: control rats that received saline for 14 days; cisplatin-treated rats that received a single intraperitoneal (i.p.) injection of cisplatin (12 mg/kg) followed by a daily oral administration of saline (0.9%) for 14 days and rats treated with a single i.p. injection of cisplatin (12 mg/kg) followed by a daily oral administration of curcumin nanoparticles (50 mg/kg) for 14 days. Cisplatin resulted in a significant increase in lipid peroxidation, nitric oxide (NO), and TNF-α and a significant decrease in reduced glutathione (GSH) levels and Na+, K+- ATPase activity. Moreover, significant increases in cardiac AchE and serum lactate dehydrogenase activities were recorded. Treatment of cisplatin-injected animals with curcumin nanoparticles ameliorated all the alterations induced by cisplatin in the heart of rats. This suggests that curcumin nanoparticles can be used as an important therapeutic adjuvant in chemotherapeutic and other toxicities mediated by oxidative stress and inflammation.




Similar content being viewed by others
References
van Laar, M., Fetbower, R. G., Gale, C. P., Bowen, D. T., Oliver, S. E., & Glaser, A. (2014). Cardiovascular sequelae in long-term survivors of young people’s cancer: A linked cohort study. British Journal of Cancer, 110, 1338–1341.
Dasari, S., & Tchounwou, P. B. (2014). Cisplatin in cancer therapy: Molecular mechanisms of action. European Journal of Pharmacology, 740, 364–378.
Barabas, K., Milner, R., Lurie, D., & Adin, C. (2008). Cisplatin: A review of toxicities and therapeutic applications. Veterinary and Comparative Oncology, 6, 1–18.
Yao, X., Panichpisal, K., Kurtzman, N., & Nugent, K. (2007). Cisplatin nephrotoxicity: A review. American Journal of the Medical Sciences, 334(2), 115–124.
Guglin, M., Aljayeh, M., Saiyad, S., Ali, R., & Curtis, A. B. (2009). Introducing a new entity: Chemotherapy-induced arrhythmia. Europace, 11, 1579–1586.
Ozcan, T., Cirit, A., & Kiykim, A. (2011). Recurrent complete atrioventricular block during cisplatin infusion: A case report. Journal of Clinical and Experimental Cardiology, 2, 151. https://doi.org/10.4172/2155-9880.1000151.
Yavas, O., Aytemir, K., & Celik, I. (2008). The prevalence of silent arrhythmia in patients receiving cisplatin-based chemotherapy. Turkish Journal of Cancer, 38, 12–15.
Bano, N., Najam, R., & Qazi, F. (2013). Adverse cardiac manifestations of cisplatin—A review. International Journal of Pharmaceutical Sciences Review and Research, 18, 80–85.
Amit, L., Ben-Aharon, I., Tichler, T., Inbar, E., Sulkes, A., & Stemmer, S. (2012). Cisplatin-induced posterior reversible encephalopathy syndrome—A brief report and review of the literature. Journal of Behavioral and Brain Science, 2, 97–101.
Ryberg, M. (2012). Recent advances in cardiotoxicity of anticancer therapies. American Society of Clinical Oncology Educational Book, 32(1), 555–559.
Wheate, N. J., Walker, S., Craig, G. E., & Oun, R. (2010). The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Transactions, 39, 8113–8127.
Zsengellér, Z. K., Ellezian, L., Brown, D., Horváth, B., Mukhopadhyay, P., Kalyanaraman, B., et al. (2012). Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. Journal of Histochemistry and Cytochemistry, 60, 521–529.
Qian, W., Nishikawa, M., Haque, A. M., Hirose, M., Mashimo, M., Sato, E., & Inoue, M. (2005). Mitochondrial density determines the cellular sensitivity to cisplatin-induced cell death. American Journal of Physiology. Cell Physiology, 289(6), C1466–C1475.
Afsar, T., Razak, S., Almajwal, A., Shabbir, M., & Khan, M. R. (2019). Evaluating the protective potency of Acacia hydaspica R. Parker on histological and biochemical changes induced by Cisplatin in the cardiac tissue of rats. BMC Complementary Medicine and Therapies, 19(1), 182.
Rosic, G., Srejovic, I., Zivkovic, V., Selakovic, D., Joksimovic, J., & Jakovljevic, V. (2015). The effects of N-acetylcysteine on cisplatin-induced cardiotoxicity onisolated rat hearts after short-term global ischemia. Toxicology Reports, 2, 996–1006.
Rajsekhar, P. B., Arvind Bharani, R. S., Jini Angel, K., Ramachandran, M., & Rajsekhar, S. P. V. (2015). Curcumin nanoparticles: A therapeutic review. RJPBCS, 6, 1180–1185.
Borra, S. K., Gurumurthy, P., & Mahendra, J. (2013). Antioxidant and free radical scavenging activity of curcumin determined by using different in vitro and ex vivo models. Journal of Medicinal Plants Research, 7(36), 2680–2690.
Carvalhd, D. D., Takeuchi, K. P., Geraldine, R. M., de Mdura, C. J., & Tdrres, M. C. L. (2015). Production, solubility and antioxidant activity of curcumin nanosuspension. Food Science and Technology (Campinas), 35(1), 115–119.
Fadus, M. C., Lau, C., Bikhchandani, J., & Lynch, H. T. (2017). Curcumin: An age-old anti-inflammatory and anti-neoplastic agent. Journal of Traditional and Complementary Medicine, 7(3), 339–346.
Bader, A. A., & Abdel Fattah, A. A. (2016). Antimicrobial activity of raw and nano turmeric powder extracts. Middle East Journal of Applied Sciences, 6(4), 787–796.
Wang, J., Wang, H. Y., Zhu, R. R., Liu, Q., Fei, J., & Wang, S. L. (2015). Anti-inflammatory activity of curcumin-loadedsolid lipid nanoparticles in IL-1 beta transgenic mice subjected to the, lipopolysaccharide-induced sepsis. Biomaterials, 53, 475–483.
Zheng, Q. T., Yang, Z. H., Yu, L. Y., Ren, Y. Y., Huang, Q. X., Liu, Q., et al. (2017). Synthesis and antioxidant activity of curcumin analogs. Journal of Asian Natural Products Research, 19, 489–503.
Jankun, J., Wyganowska-Swiatkowska, M., Dettlaff, K., Jelinska, A., Surdacka, A., Watrobska-Swietlikowska, D., & Skrzypczak-Jankun, E. (2016). Determining whether curcumin degradation/condensation is actually bioactivation (review). International Journal of Molecular Medicine, 37, 1151–1158.
Adahoun, M. A., Al-Akhras, M. A. H., Jaafar, M. S., & Bououdina, M. (2017). Enhanced anti-cancer and antimicrobial activities of curcumin nanoparticles. Artificial Cells, Nanomedicine, and Biotechnology, 45, 98–107.
Khayyal, M. T., El-Hazek, R. M., El-Sabbagh, W. A., Frank, J., Behnam, D., & Abdel-Tawab, M. (2018). Micellar solubilisation enhances the antiinflammatory activities of curcumin and boswellic acids in rats with adjuvant-induced arthritis. Nutrition, 54, 189–196.
Akbar, M. U., Zia, K. M., Nazir, A., Iqbal, J., Ejaz, S. A., & Akash, M. S. H. (2018). Pluronic-based mixed polymeric micelles enhance the therapeutic potential of curcumin. An Official Journal of the American Association of Pharmaceutical Scientists, 19, 2719–2739.
Kakkar, V., Mishra, A. K., Chuttani, K., & Kaur, I. P. (2013). Proof of concept studies to confirm the delivery of curcumin loaded solid lipid nanoparticles (C-SLNs) to brain. International Journal of Pharmaceutics, 448, 354–359.
Sankar, P., Telang, A. G., Suresh, S., Kesavan, M., Kannan, K., Kalaivanan, R., & Sarkar, S. N. (2013). Immunomodulatory effects of nanocurcumin in arsenic-exposed rats. International Immunopharmacology, 17, 65–70.
Khadrawy, Y. A., El-Gizawy, M. M., Sorour, S. M., Sawie, H. G., & Hosny, E. N. (2019). Effect of curcumin nanoparticles on the cisplatin-induced neurotoxicity in rat. Drug and Chemical Toxicology, 42, 194–202.
Carlson, L. J., Cote, B., Alani, A. W., & Rao, D. A. (2014). Polymeric micellar codelivery of resveratrol and curcumin to mitigate in vitro doxorubicin-induced cardiotoxicity. Journal of Pharmaceutical Sciences, 103, 2315–2322.
Li, J., Gye, G. H., Chen, X., & Park, H. J. (2015). Modified curcumin with hyaluronic acid: Combination of pro-drug and nano-micelle strategy to address the curcumin challenge. Food Research International, 69, 202–208.
Ismaiel, A. A. M., El-Denshary, E. S., El-Nekkety, A. A., Al-Yamani, A. F., Gad, A. S., & Hassan, N. S. (2015). Ameliorative effects of curcumin nanoparticles on hepatotoxicity induced by Zearalenone Mycotoxin. Global Journal of Pharmacology, 9, 234–245.
Abas, A. M. (2017). Evaluation of the protective effects of ginger extract on cisplatin induced cardiotoxicity in male albino rats. Journal of Chemical and Pharmaceutical Research, 9(2), 99–110.
Ruiz-Larrea, M. B., Leal, A. M., Liza, M., Lacort, M., & de Groot, H. (1994). Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids, 59, 383–388.
Montgomery, H. A. C., & Dymock, J. F. (1961). The determination of nitrite in water. Analyst, 86, 414–416.
Beutler, E., Duron, O., & Kelly, B. M. (1963). Improved method for the determination of blood glutathione. Journal of Laboratory and Clinical Medicine, 61, 882–888.
Ellman, G. L., Courtney, K. D., Andres, V., & Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7, 88–95.
Gorun, V., Proinov, I., Baltescu, V., Balaban, G., & Barzu, O. (1978). Modified Ellman procedure for assay of cholinesterase in crude-enzymatic preparations. Analytical Biochemistry, 86, 324–326.
Tsakiris, S., Angelogianni, P., Schulpis, K. H., & Behrakis, P. (2000). Protective effect of l-cysteine and glutathione on rat brain Na+, K+ ATPase inhibition induced by free radicals. Zeitschrift für Naturforschung C, 55, 271–277.
Bais, R., & Philcox, M. (1994). Approved recommendation on IFCC methods for the measurement of catalytic concentration of enzymes. Part 8. IFCC method for lactate dehydrogenase (l-lactate: NAD+Oxidoreductase, EC 1.1.1.27). International Federation of Clinical Chemistry (IFCC). European Journal of Clinical Chemistry and Clinical Biochemistry, 32, 639–655.
Topal, İ, Bilgin, A. Ö., Çimen, F. K., Kurt, N., Süleyman, Z., Bilgin, Y., et al. (2018). The effect of rutin on cisplatin-induced oxidative cardiac damage in rats. The Anatolian Journal of Cardiology, 20, 136–142.
El-SE, E.-A., Moustafa, Y. M., Abo-Elmatty, D. M., & Radwan, A. (2011). Cisplatin-induced cardiotoxicity: Mechanisms and cardioprotective strategies. European Journal of Pharmacology, 650, 335–3341.
Lomeli, N., Di, K., Czerniawski, J., Guzowski, J. F., & Bota, D. A. (2017). Cisplatin-induced mitochondrial dysfunction is associated with impaired cognitive function in rats. Free Radical Biology and Medicine, 102, 274–286.
Dzagnidze, A., Katsarava, Z., Makhalova, J., Liedert, B., Yoon, M. S., Kaube, H., et al. (2007). Repair capacity for platinum-DNA adducts determines the severity of cisplatin-induced peripheral neuropathy. Journal of Neuroscience, 27, 9451–9457.
Wu, G., Fang, Y. Z., Yang, S., Lupton, J. R., & Turner, N. D. (2004). Glutathione metabolism and its implications for health. Journal of Nutrition, 134, 489–492.
Ferdinandy, P., & Schulz, R. (2003). Nitric oxide, superoxide, and peroxynitrite in myocardial ischaemia-reperfusion injury and preconditioning. British Journal of Pharmacology, 138, 532–543.
Fuller, W., Tulloch, L. B., Shattock, M. J., Calaghan, S. C., Howie, J., & Wypijewski, K. J. (2013). Regulation of the cardiac sodium pump. Cellular and Molecular Life Sciences, 70, 1357–1380.
Bossuyt, J., Ai, X., Moorman, J. R., Pogwizd, S. M., & Bers, D. M. (2005). Expression and phosphorylation of the Na-pump regulatory subunit phospholemman in heart failure. Circulation Research, 97, 558–565.
Workman, A. J., Kane, K. A., & Rankin, A. C. (2013). Characterisation of the Na, K pump current in atrial cells from patients with and without chronic atrial fibrillation. Cardiovascular Research, 59, 593–602.
Dostanic-Larson, I., van Huysse, J. W., Lorenz, J. N., & Lingrel, J. B. (2005). The highly conserved cardiac glycoside binding site of Na+, K+-ATPase plays a role in blood pressure regulation. Proceedings of the National academy of Sciences of the United States of America, 102, 15845–15850.
Fuller, W., Eaton, P., Bell, J. R., & Shattock, M. J. (2004). Ischemia-induced phosphorylation of phospholemman directly activates rat cardiac Na+/K+ ATPase. The FASEB Journal, 18, 197–199.
Bibert, S., Liu, C. C., Figtree, G. A., Garcia, A., Hamilton, E. J., Marassi, F. M., et al. (2011). XYD proteins reverse inhibition of the Na+-K+ pump mediated by glutathionylation of its β1 subunit. Journal of Biological Chemistry, 286, 18562–18572.
Reifenberger, M. S., Arnett, K. L., Gatto, C., & Milanick, M. A. (2008). The reactive nitrogen species peroxynitrite is a potent inhibitor of renal Na+-K+-ATPase activity. American Journal of Physiology. Renal Physiology, 295, F1191-1198.
Xi, H. J., Wu, R. P., Liu, J. J., Zhang, L. J., & Li, Z. S. (2015). Role of acetylcholinesterase in lung cancer. Thoracic Cancer, 6(4), 390–398.
Sperling, L. E., Steinert, G., Boutter, J., Landgraf, D., Hescheler, J., Pollet, D., & Layer, P. G. (2008). Characterisation of cholinesterase expression during murine embryonic stem cell differentiation. Chemico-Biological Interactions, 175, 156–160.
Park, S. E., & Yoo, Y. H. (2010). Acetylcholinesterase as a pharmacological target in cancer research. In F. Cecconi & M. D’Amelio (Eds.), Apoptosome: An up-and-coming therapeutical tool (pp. 221–236). Dordrecht: Springer.
Olofsson, P. S., Rosas-Ballina, M., Levine, Y. A., & Tracey, K. J. (2012). Rethinking inflammation: Neural circuits in the regulation of immunity. Immunological Reviews, 248, 188–204.
Adamy, C., Le Corvoisier, P., Candiani, G., Kirsch, M., Pavoine, C., Defer, N., et al. (2005). Tumor necrosis factor alpha and glutathione interplay in chronic heart failure. Archives des Maladies du Coeur et des Vaisseaux, 98, 906–912.
Ping, Z., Aiqun, M., Jiwu, L., & Liang, S. (2017). TNF receptor 1/2 predict heart failure risk in type 2 diabetes mellitus patients. International Heart Journal, 58, 245–249.
Staal, F. J., Roederer, M., & Herzenberg, L. A. (1990). Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proceedings of the National academy of Sciences of the United States of America, 87, 9943–9947.
Haddad, J. J. (2002). Redox regulation of pro-inflammatory cytokines and IkappaB-alpha/NF-kappaB nuclear translocation and activation. Biochemical and Biophysical Research Communications, 296, 847–856.
Anand, P., Kunnukakkara, A. B., Newman, R. A., & Aggarwal, B. B. (2007). Bioavailability of curcumin: Problems and promises. Molecular Pharmaceutics, 4, 807–818.
Ravichandran, R. (2013). Pharmacokinetic study of nanoparticulate curcumin: Oral formulation for enhanced bioavailability. JBNB, 4, 291–299.
Ma, Z., Shayeganpour, A., Brocks, D. R., Lavasanifar, A., & Samuel, J. (2007). High-performance liquid chromatography analysis of curcuminin rat plasma: Application to pharmacokinetics of polymeric micellar formulation of curcumin. Biomedical Chromatography, 21, 546–552.
Flora, G., Gupta, D., & Tiwari, A. (2013). Nanocurcumin: A promising therapeutic advancement over native curcumin. Critical Reviews in Therapeutic Drug Carrier Systems, 30, 331–368.
Nehra, S., Bhardwaj, V., Kalra, N., Ganju, L., Bansal, A., Saxena, S., & Saraswat, D. (2015). Nanocurcumin protects cardiomyoblasts H9c2 from hypoxia-induced hypertrophy and apoptosis by improving oxidative balance. Journal of Physiology and Biochemistry, 71, 239–251.
Tyagi, P., Singh, M., Kumari, H., Kumari, A., & Mukhopadhyay, K. (2015). Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS ONE, 10, e0121313.
Abo-Salem, O. M., Harisa, G. I., Ali, T. M., El-Sayed, S. M., & Abou-Elnour, F. M. (2014). Curcumin ameliorates streptozotocin-induced heart injury in rats. Journal of Biochemical and Molecular Toxicology, 28, 263–270.
Rahmi, D. N. I., Louisa, M., & Soetikno, V. (2018). Effects of curcumin and nanocurcumin on cisplatin-induced nephrotoxicity in rat: Copper transporter 1 and organic cation transporter 2 as drug transporters. International Journal of Applied Pharmaceutics, 10, 172–174.
Lestari, M. L., & Indrayanto, G. (2014). Curcumin. Profiles of Drug Substances, Excipients and Related Methodology, 39, 113–204.
Setyono, J., Harini, I. M., Sarmoko, S., & Rujito, L. (2019). Supplementation of curcuma domestica extract reduces cox-2 and inos expression on raw 2647 cells. Journal of Physics: Conference Series, 1246, 012059.
Singh, P., Kesharwani, R. K., Misra, K., & Rizvi, S. I. (2015). The modulation of erythrocyte Na+/K+-ATPaseactivity by curcumin. Journal of Advanced Research, 6, 1023–1030.
Orhan, I. E. (2013). Nature: A substantial source of auspicious substances with acetylcholinesterase inhibitory action. Current Neuropharmacology, 11, 379–387.
Tiwari, V., & Chopra, K. (2013). Protective effect of curcumin against chronic alcohol-induced cognitive deficits and neuroinflammation in the adult rat brain. Neuroscience, 6, 147–158.
Yuan, J., Liu, R., Ma, Y., Zhang, Z., & Xie, Z. (2018). Curcumin attenuates airway inflammation and airway remolding by inhibiting NF-kB signaling and COX-2 in cigarette smoke-induced COPD mice. Inflammation, 41, 1804–1814.
Rocha, B. A., Gonçalves, O. H., Leimann, F. V., Rebecca, E. S. W., Silva-Buzanello, R. A., Filho, L. C., et al. (2014). Curcumin encapsulated in poly-L-lactic acid improves its anti-inflammatory efficacy in vivo. Advancement in Medicinal Plant Research, 2(4), 62–73.
Ghandadi, M., & Sahebkar, A. (2017). Curcumin: An effective inhibitor of interleukin-6. Current Pharmaceutical Design, 23, 921–931.
Pan, Y., Zhao, D., Yu, N., An, T., Miao, J., Mo, F., et al. (2017). Curcumin improves glycolipid metabolism through regulating peroxisome proliferator activated receptor γ signalling pathway in high-fat diet-induced obese mice and 3T3-L1 adipocytes. Royal Society Open Science, 4, 170917.
Funding
No funding was obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Animal procedures were approved by the Institutional Animal Care and Use Committee of Cairo University, Faculty of Science (IACUC) (Egypt), (CU/I/F/42/19).
Additional information
Handling Editor: Y. Robert Li.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khadrawy, Y.A., Hosny, E.N., El-Gizawy, M.M. et al. The Effect of Curcumin Nanoparticles on Cisplatin-Induced Cardiotoxicity in Male Wistar Albino Rats. Cardiovasc Toxicol 21, 433–443 (2021). https://doi.org/10.1007/s12012-021-09636-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09636-3