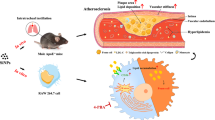
Abstract
Ultrafine particles (UFPs) referred to particular matters with aerosol diameter less than 100 nm. Because of the lightweight and small size, UFPs have become an occupational inhalation risk. The UFPs will be accumulated in the deep lung through inhalation, and then reach into all the organs via circulation system; some UFPs even stay in the brain. As previous study reported, UFPs exposure is usually associated with cardiovascular disease, such as atherosclerosis (AS). In our study, we tried to understand how acute UFP exposure caused the biological dysregulation in atherosclerosis. Acute exposure of UFPs were applied to mice for 6 consecutive days, mice were sacrificed after 3, 5, 7, and 10 days post-exposure. Aorta and serum were collected for histological and biomarkers analysis. Mice aortic adventitial fibroblasts (MAFs) were isolated from mice and used to further study to understand the mechanism of UFPs induced atherosclerosis. Compared to the untreated control, the inflammation responses and nitrate stress were observed after acute exposure of UFPs, with increased IL-6, MCP-1, p47phox, and 3-NT levels in the mice serum. Besides, upregulation of microRNAs: miR-301b-3p and Let-7c-1-3p, and their downstream target: Smad2, Smad3, and TGFβ1 were also observed in mouse aorta after acute exposure of UFPs. Similar results were identified in vitro as well. Acute exposure of UFPs induced the systematic nitrate stress and inflammation responses, along with the changes of vascular permeability. Dysregulated miRNAs and TGFβ/Smads signaling pathway indicated the higher risk of atherosclerosis/vasculature remodeling when exposed to UFPs.






Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Morawska, L., Ristovski, Z., Jayaratne, E., Keogh, D. U., & Ling, X. (2008). Ambient nano and ultrafine particles from motor vehicle emissions: Characteristics, ambient processing and implications on human exposure. Atmospheric Environment, 42(35), 8113–8138.
Chen, R., Hu, B., Liu, Y., Xu, J., Yang, G., Xu, D., et al. (2016). Beyond PM2.5: The role of ultrafine particles on adverse health effects of air pollution. Biochimica Biophysica Acta, 1860(12), 2844–2855. https://doi.org/10.1016/j.bbagen.2016.03.019.
Calderon-Garciduenas, L., Engle, R., Mora-Tiscareno, A., Styner, M., Gomez-Garza, G., Zhu, H., et al. (2011). Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain and Cognition, 77(3), 345–355. https://doi.org/10.1016/j.bandc.2011.09.006.
Costello, S., Brown, D. M., Noth, E. M., Cantley, L., Slade, M. D., Tessier-Sherman, B., et al. (2014). Incident ischemic heart disease and recent occupational exposure to particulate matter in an aluminum cohort. Journal of Exposure Science & Environmental Epidemiology, 24(1), 82–88. https://doi.org/10.1038/jes.2013.47.
Farina, F., Lonati, E., Milani, C., Massimino, L., Ballarini, E., Donzelli, E., et al. (2019). In vivo comparative study on acute and sub-acute biological effects induced by ultrafine particles of different anthropogenic sources in BALB/c mice. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms20112805.
Downward, G. S., van Nunen, E., Kerckhoffs, J., Vineis, P., Brunekreef, B., Boer, J. M. A., et al. (2018). Long-term exposure to ultrafine particles and incidence of cardiovascular and cerebrovascular disease in a prospective study of a Dutch cohort. Environmental Health Perspectives, 126(12), 127007. https://doi.org/10.1289/ehp3047.
Cascio, W. E., Cozzi, E., Hazarika, S., Devlin, R. B., Henriksen, R. A., Lust, R. M., et al. (2007). Cardiac and vascular changes in mice after exposure to ultrafine particulate matter. Inhalation Toxicology, 19(Suppl 1), 67–73. https://doi.org/10.1080/08958370701493456.
Kilinc, E., Van Oerle, R., Borissoff, J. I., Oschatz, C., Gerlofs-Nijland, M. E., Janssen, N. A., et al. (2011). Factor XII activation is essential to sustain the procoagulant effects of particulate matter. Journal of Thrombosis and Haemostasis, 9(7), 1359–1367. https://doi.org/10.1111/j.1538-7836.2011.04280.x.
Bräuner, E. V., Forchhammer, L., Møller, P., Simonsen, J., Glasius, M., Wåhlin, P., et al. (2007). Exposure to ultrafine particles from ambient air and oxidative stress–induced DNA damage. Environmental Health Perspectives, 115(8), 1177–1182.
Rychlik, K. A., Secrest, J. R., Lau, C., Pulczinski, J., Zamora, M. L., Leal, J., et al. (2019). In utero ultrafine particulate matter exposure causes offspring pulmonary immunosuppression. Proceedings of the National Academy of Sciences, 116(9), 3443–3448. https://doi.org/10.1073/pnas.1816103116.
Mahley, R. W. (2016). Apolipoprotein E: From cardiovascular disease to neurodegenerative disorders. Journal of Molecular Medicine (Berl), 94(7), 739–746. https://doi.org/10.1007/s00109-016-1427-y.
Okamoto, Y., Kihara, S., Ouchi, N., Nishida, M., Arita, Y., Kumada, M., et al. (2002). Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation, 106(22), 2767–2770.
Balasubramanian, S. K., Poh, K.-W., Ong, C.-N., Kreyling, W. G., Ong, W.-Y., & Liya, E. Y. (2013). The effect of primary particle size on biodistribution of inhaled gold nano-agglomerates. Biomaterials, 34(22), 5439–5452.
Gradinaru, D., Borsa, C., Ionescu, C., & Prada, G. I. (2015). Oxidized LDL and NO synthesis–Biomarkers of endothelial dysfunction and ageing. Mechanisms of Ageing and Development, 151, 101–113. https://doi.org/10.1016/j.mad.2015.03.003.
Suhaimi, N. F., & Jalaludin, J. (2015). Biomarker as a research tool in linking exposure to air particles and respiratory health. BioMed Research International, 2015, 962853. https://doi.org/10.1155/2015/962853.
Radi, R. (2004). Nitric oxide, oxidants, and protein tyrosine nitration. Proceedings of the National Academy of Sciences, 101(12), 4003–4008.
Perrone, S., Tataranno, M. L., Santacroce, A., Negro, S., & Buonocore, G. (2014). The role of oxidative stress on necrotizing enterocolitis in very low birth weight infants. Current Pediatric Reviews, 10(3), 202–207.
Jeong, A., Fiorito, G., Keski-Rahkonen, P., Imboden, M., Kiss, A., Robinot, N., et al. (2018). Perturbation of metabolic pathways mediates the association of air pollutants with asthma and cardiovascular diseases. Environment International, 119, 334–345.
Kuijpers, E., Pronk, A., Kleemann, R., Vlaanderen, J., Lan, Q., Rothman, N., et al. (2018). Cardiovascular effects among workers exposed to multiwalled carbon nanotubes. Occupational and Environmental Medicine, 75(5), 351–358.
Li, R., Ning, Z., Cui, J., Yu, F., Sioutas, C., & Hsiai, T. (2010). Diesel exhaust particles modulate vascular endothelial cell permeability: Implication of ZO-1 expression. Toxicology Letters, 197(3), 163–168.
Shi, Y., Zhao, T., Yang, X., Sun, B., Li, Y., Duan, J., et al. (2019). PM2. 5-induced alteration of DNA methylation and RNA-transcription are associated with inflammatory response and lung injury. Science of The Total Environment, 650, 908–921.
Wang, Q., Masud, A., Aich, N., & Wu, Y. (2018). In vitro pulmonary toxicity of reduced graphene oxide-nano zero valent iron nanohybrids and comparison with parent nanomaterial attributes. ACS Sustainable Chemistry & Engineering, 6(10), 12797–12806.
Wang, Q., Khan, N. A., Muthumalage, T., Lawyer, G. R., McDonough, S. R., Chuang, T. D., et al. (2019). Dysregulated repair and inflammatory responses by e-cigarette-derived inhaled nicotine and humectant propylene glycol in a sex-dependent manner in mouse lung. FASEB Bioadvances, 1(10), 609–623. https://doi.org/10.1096/fba.2019-00048.
Alinovi, R., Goldoni, M., Pinelli, S., Campanini, M., Aliatis, I., Bersani, D., et al. (2015). Oxidative and pro-inflammatory effects of cobalt and titanium oxide nanoparticles on aortic and venous endothelial cells. Toxicology in Vitro, 29(3), 426–437. https://doi.org/10.1016/j.tiv.2014.12.007.
An, Z., Jin, Y., Li, J., Li, W., & Wu, W. (2018). Impact of particulate air pollution on cardiovascular health. Current Allergy and Asthma Reports, 18(3), 15.
Ji, X., Zhang, Y., Li, G., & Sang, N. (2018). Potential role of inflammation in associations between particulate matter and heart failure. Current Pharmaceutical Design, 24(3), 341–358.
Bai, N., Kido, T., Suzuki, H., Yang, G., Kavanagh, T. J., Kaufman, J. D., et al. (2011). Changes in atherosclerotic plaques induced by inhalation of diesel exhaust. Atherosclerosis, 216(2), 299–306. https://doi.org/10.1016/j.atherosclerosis.2011.02.019.
Weldy, C. S., Liu, Y., Liggitt, H. D., & Chin, M. T. (2014). In utero exposure to diesel exhaust air pollution promotes adverse intrauterine conditions, resulting in weight gain, altered blood pressure, and increased susceptibility to heart failure in adult mice. PLoS ONE, 9(2), e88582. https://doi.org/10.1371/journal.pone.0088582.
Brennan, E., Wang, B., McClelland, A., Mohan, M., Marai, M., Beuscart, O., et al. (2017). Protective effect of let-7 miRNA family in regulating inflammation in diabetes-associated atherosclerosis. Diabetes, 66(8), 2266–2277. https://doi.org/10.2337/db16-1405.
Parahuleva, M. S., Lipps, C., Parviz, B., Hölschermann, H., Schieffer, B., Schulz, R., et al. (2018). MicroRNA expression profile of human advanced coronary atherosclerotic plaques. Scientific Reports, 8(1), 7823.
Liu, R.-M., & Pravia, K. G. (2010). Oxidative stress and glutathione in TGF-β-mediated fibrogenesis. Free Radical Biology and Medicine, 48(1), 1–15.
Khaliullin, T. O., Kisin, E. R., Murray, A. R., Yanamala, N., Shurin, M. R., Gutkin, D. W., et al. (2017). Mediation of the single-walled carbon nanotubes induced pulmonary fibrogenic response by osteopontin and TGF-β1. Experimental Lung Research, 43(8), 311–326.
Chen, Z., Wang, Q., Asmani, M., Li, Y., Liu, C., Li, C., et al. (2016). Lung microtissue array to screen the fibrogenic potential of carbon nanotubes. Scientific Reports, 6, 31304.
Lund, A. K., Lucero, J., Lucas, S., Madden, M. C., McDonald, J. D., Seagrave, J. C., et al. (2009). Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1-mediated pathways. Arteriosclerosis, Thrombosis, and Vascular Biology, 29(4), 511–517. https://doi.org/10.1161/atvbaha.108.176107.
Lund, A. K., Knuckles, T. L., Obot Akata, C., Shohet, R., McDonald, J. D., Gigliotti, A., et al. (2006). Gasoline exhaust emissions induce vascular remodeling pathways involved in atherosclerosis. Toxicological Sciences, 95(2), 485–494.
Funding
This work was financially supported by National Natural Science Foundation of China (81803270) and National Key Research and Development Program of China (2016YFC0206900).
Author information
Authors and Affiliations
Contributions
KL, JY, XL and ZX carried out the studies, participated in collecting data, and drafted the manuscript. SW and XL, performed the statistical analysis and participated in its design. BL, LT and HL participated in acquisition, analysis, or interpretation of data and draft the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Research and animal care procedures were approved by the Animal and Human Use in Research Committee of the Tianjin Institute of Environmental and Operational Medicine, and all animal experiments were performed in accordance with relevant guidelines and regulations.
Additional information
Handling Editor: Phillip G. Kopf.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, K., Yan, J., Wang, S. et al. Acute Exposure of Atmospheric Ultrafine Particles Induced Inflammation Response and Dysregulated TGFβ/Smads Signaling Pathway in ApoE−/− Mice. Cardiovasc Toxicol 21, 410–421 (2021). https://doi.org/10.1007/s12012-021-09633-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09633-6