Abstract
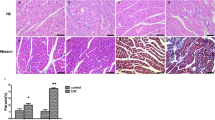
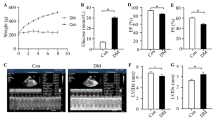
Emerging evidence shows that the transient receptor potential vanilloid 4 (TRPV4) channel is involved in fibrosis in many organs. However, its role in diabetic cardiac fibrosis remains unclear. Our aim was to evaluate the expression level of TRPV4 in the diabetic heart and clarify its role in diabetes-induced cardiac fibrosis. A diabetic animal model was induced by a single intraperitoneal injection of streptozotocin into Sprague–Dawley rats. We also investigated cardiac fibroblasts isolated from neonatal Sprague–Dawley rats. TRPV4 expression was significantly upregulated in both diabetic myocardium and cardiac fibroblasts cultured in high-glucose medium. Masson’s trichrome staining revealed that the TRPV4 antagonist HC067047 attenuated the diabetes-induced cardiac fibrosis. Furthermore, HC067047 reduced collagen Ι synthesis and suppressed the transforming growth factor beta 1 (TGF-β1) level as well as the phosphorylation of Smad3 in the diabetic heart. In addition, the TRPV4 antagonist inhibited the proliferation of cardiac fibroblasts, collagen Ι synthesis, and activation of the TGF-β1/Smad3 signaling pathway induced by high-glucose culture medium. Our findings demonstrate that the upregulation of TRPV4 expression mediates diabetic cardiac fibrosis via activation of the TGF-β1/Smad3 signaling pathway.






Similar content being viewed by others
Abbreviations
- DM:
-
Diabetes mellitus
- TRPV:
-
Transient receptor potential vanilloid
- ECM:
-
Extracellular matrix
- CFs:
-
Cardiac fibroblasts
- STZ:
-
Streptozotocin
References
Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., Cushman, M., et al. (2016). Heart disease and stroke statistics-2016 Update: A report from the American Heart Association. Circulation, 133(4), e38–360. https://doi.org/10.1161/CIR.0000000000000350.
Plutzky, J. (2011). Macrovascular effects and safety issues of therapies for type 2 diabetes. The American Journal of Cardiology, 108(3 Suppl), 25B–32B. https://doi.org/10.1016/j.amjcard.2011.03.014.
Shao, S., Zhang, X., Duan, L., Fang, H., Rao, S., Liu, W., et al. (2018). Lysyl Hydroxylase Inhibition by Minoxidil Blocks Collagen Deposition and Prevents Pulmonary Fibrosis via TGF-beta(1)/Smad3 Signaling Pathway. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 24, 8592–8601. https://doi.org/10.12659/MSM.910761.
Adameova, A., & Dhalla, N. S. (2014). Role of microangiopathy in diabetic cardiomyopathy. Heart Failure Reviews, 19(1), 25–33. https://doi.org/10.1007/s10741-013-9378-7.
Joshi, M., Kotha, S. R., Malireddy, S., Selvaraju, V., Satoskar, A. R., Palesty, A., et al. (2014). Conundrum of pathogenesis of diabetic cardiomyopathy: Role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria. Molecular and Cellular Biochemistry, 386(1–2), 233–249. https://doi.org/10.1007/s11010-013-1861-x.
Ather, S., Chan, W., Bozkurt, B., Aguilar, D., Ramasubbu, K., Zachariah, A. A., et al. (2012). Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. Journal of the American College of Cardiology, 59(11), 998–1005. https://doi.org/10.1016/j.jacc.2011.11.040.
Paulus, W. J., & Tschope, C. (2013). A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology, 62(4), 263–271. https://doi.org/10.1016/j.jacc.2013.02.092.
Chacar, S., Fares, N., Bois, P., & Faivre, J.-F. (2017). Basic Signaling in Cardiac Fibroblasts. Journal of Cellular Physiology, 232(4), 725–730. https://doi.org/10.1002/jcp.25624.
Camelliti, P., Borg, T. K., & Kohl, P. (2005). Structural and functional characterisation of cardiac fibroblasts. Cardiovascular Research, 65(1), 40–51. https://doi.org/10.1016/j.cardiores.2004.08.020.
Liu, Y., Qi, H., Mingyao, E., Shi, P., Zhang, Q., Li, S., et al. (2018). Transient receptor potential vanilloid-3 (TRPV3) activation plays a central role in cardiac fibrosis induced by pressure overload in rats via TGF-beta1 pathway. Naunyn Schmiedebergs Archives of Pharmacology, 391(2), 131–143. https://doi.org/10.1007/s00210-017-1443-7.
Ruppert, M., Bodi, B., Korkmaz-Icoz, S., Loganathan, S., Jiang, W., Lehmann, L., et al. (2019). Myofilament Ca(2+) sensitivity correlates with left ventricular contractility during the progression of pressure overload-induced left ventricular myocardial hypertrophy in rats. Journal of Molecular and Cellular Cardiology, 129, 208–218. https://doi.org/10.1016/j.yjmcc.2019.02.017.
Roe, A. T., Aronsen, J. M., Skardal, K., Hamdani, N., Linke, W. A., Danielsen, H. E., et al. (2017). Increased passive stiffness promotes diastolic dysfunction despite improved Ca2+ handling during left ventricular concentric hypertrophy. Cardiovascular Research, 113(10), 1161–1172. https://doi.org/10.1093/cvr/cvx087.
Suarez, J., Cividini, F., Scott, B. T., Lehmann, K., Diaz-Juarez, J., Diemer, T., et al. (2018). Restoring mitochondrial calcium uniporter expression in diabetic mouse heart improves mitochondrial calcium handling and cardiac function. The Journal of Biological Chemistry, 293(21), 8182–8195. https://doi.org/10.1074/jbc.RA118.002066.
Zhang, L., Ward, M.-L., Phillips, A. R. J., Zhang, S., Kennedy, J., Barry, B., et al. (2013). Protection of the heart by treatment with a divalent-copper-selective chelator reveals a novel mechanism underlying cardiomyopathy in diabetic rats. Cardiovascular Diabetology, 12, 123. https://doi.org/10.1186/1475-2840-12-123.
Choi, K. M., Zhong, Y., Hoit, B. D., Grupp, I. L., Hahn, H., Dilly, K. W., et al. (2002). Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in Type 1 diabetic rats. American Journal of Physiology Heart and Circulatory Physiology, 283(4), H1398–H1408. https://doi.org/10.1152/ajpheart.00313.2002.
Li, H. (2017). TRP channel classification. Advances in Experimental Medicine and Biology, 976, 1–8. https://doi.org/10.1007/978-94-024-1088-4_1.
Adapala, R. K., Thoppil, R. J., Luther, D. J., Paruchuri, S., Meszaros, J. G., Chilian, W. M., et al. (2013). TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. Journal of Molecular and Cellular Cardiology, 54, 45–52. https://doi.org/10.1016/j.yjmcc.2012.10.016.
Liu, Y., Qi, H., Miangyao, E., Shi, P., Zhang, Q., Li, S.,& Sun, H., (2018). Transient receptor potential vanilloid-3 (TRPV3) activation plays a central role in cardiac fibrosis induced by pressure overload in rats via TGF-beta1 pathway. Naunyn-Schmiedeberg’s Archives of Pharmacology, 391(2), 131–143. https://doi.org/10.1007/s00210-017-1443-7.
Heymann, H. M., Wu, Y., Lu, Y., Qvit, N., Gross, G. J., & Gross, E. R. (2017). Transient receptor potential vanilloid 1 inhibitors block laparotomy- and opioid-induced infarct size reduction in rats. British Journal of Pharmacology, 174(24), 4826–4835. https://doi.org/10.1111/bph.14064.
Filosa, J. A., Yao, X., & Rath, G. (2013). TRPV4 and the regulation of vascular tone. Journal of Cardiovascular Pharmacology, 61(2), 113–119. https://doi.org/10.1097/FJC.0b013e318279ba42.
Darby, W. G., Grace, M. S., Baratchi, S., & McIntyre, P. (2016). Modulation of TRPV4 by diverse mechanisms. The International Journal of Biochemistry & Cell Biology, 78, 217–228. https://doi.org/10.1016/j.biocel.2016.07.012.
Martinac, B., & Poole, K. (2018). Mechanically activated ion channels. The International Journal of Biochemistry & Cell Biology, 97, 104–107. https://doi.org/10.1016/j.biocel.2018.02.011.
Randhawa, P. K., & Jaggi, A. S. (2015). TRPV4 channels: Physiological and pathological role in cardiovascular system. Basic Research in Cardiology, 110(6), 54. https://doi.org/10.1007/s00395-015-0512-7.
Wu, Q.-F., Qian, C., Zhao, N., Dong, Q., Li, J., Wang, B.-B., et al. (2017). Activation of transient receptor potential vanilloid 4 involves in hypoxia/reoxygenation injury in cardiomyocytes. Cell Death & Disease, 8(5), e2828. https://doi.org/10.1038/cddis.2017.227.
Dong, Q., Li, J., Wu, Q.-F., Zhao, N., Qian, C., Ding, D., et al. (2017). Blockage of transient receptor potential vanilloid 4 alleviates myocardial ischemia/reperfusion injury in mice. Scientific Reports, 7, 42678. https://doi.org/10.1038/srep42678.
Zhan, L., & Li, J. (2018). The role of TRPV4 in fibrosis. Gene, 642, 1–8. https://doi.org/10.1016/j.gene.2017.10.067.
Rahaman, S. O., Grove, L. M., Paruchuri, S., Southern, B. D., Abraham, S., Niese, K. A., et al. (2014). TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. The Journal of Clinical Investigation, 124(12), 5225–5238. https://doi.org/10.1172/JCI75331.
Gombedza, F., Kondeti, V., Al-Azzam, N., Koppes, S., Duah, E., Patil, P., et al. (2017). Mechanosensitive transient receptor potential vanilloid 4 regulates Dermatophagoides farinae-induced airway remodeling via 2 distinct pathways modulating matrix synthesis and degradation. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology, 31(4), 1556–1570. https://doi.org/10.1096/fj.201601045R.
Songa, Y., Zhan, L., Yu, M., Huang, C., Meng, X., Taotao, M., et al. (2014). TRPV4 channel inhibits TGF-β1-induced proliferation of hepatic stellate cells. PLoS ONE, 9(7), 1–10. https://doi.org/10.1371/journal.pone.0101179.
Feng, B., Chen, S., George, B., Feng, Q., & Chakrabarti, S. (2010). miR133a regulates cardiomyocyte hypertrophy in diabetes. Diabetes/Metabolism Research and Reviews, 26(1), 40–49. https://doi.org/10.1002/dmrr.1054.
Shen, E., Diao, X., Wang, X., Chen, R., & Hu, B. (2011). MicroRNAs involved in the mitogen-activated protein kinase cascades pathway during glucose-induced cardiomyocyte hypertrophy. The American Journal of Pathology, 179(2), 639–650. https://doi.org/10.1016/j.ajpath.2011.04.034.
Hu, H.-H., Chen, D.-Q., Wang, Y.-N., Feng, Y.-L., Cao, G., Vaziri, N. D., et al. (2018). New insights into TGF-beta/Smad signaling in tissue fibrosis. Chemico-Biological Interactions, 292, 76–83. https://doi.org/10.1016/j.cbi.2018.07.008.
Acknowledgements
We thank Weifeng Huang for excellent technical assistance and Dr. IC Bruce for reading the manuscript. This research was supported by grants from the National Natural Science Foundation of China (30872716), the Natural Science Foundation of Hubei Province (2015CFB288), and a Health and Family Planning Project in Hubei Province (WJ2015MB171).
Author information
Authors and Affiliations
Contributions
XLJ and CX carried out the experiments and drafted the manuscript. DQS was involved in data analysis. MCY, QYC, and JW contributed to conducting the experiments. SZZ conceived the study, reviewed the data, and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Y. James Kang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jia, X., Xiao, C., Sheng, D. et al. TRPV4 Mediates Cardiac Fibrosis via the TGF-β1/Smad3 Signaling Pathway in Diabetic Rats. Cardiovasc Toxicol 20, 492–499 (2020). https://doi.org/10.1007/s12012-020-09572-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-020-09572-8