Abstract
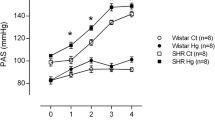
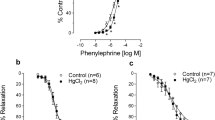
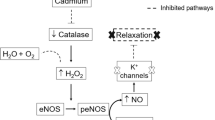
Heavy metal exposure is associated with cardiovascular diseases such as myocardial infarction (MI). Vascular dysfunction is related to both the causes and the consequences of MI. We investigated whether chronic exposure to low doses of mercury chloride (HgCl2) worsens MI-induced endothelial dysfunction 7 days after MI. Male Wistar rats were divided into four groups: Control (vehicle), HgCl2 (4 weeks of exposure), surgically induced MI and combined HgCl2-MI. Morphological and hemodynamic measurements were used to characterize the MI model 7 days after the insult. Vascular reactivity was evaluated in aortic rings. Chronic HgCl2 exposure did not cause more heart injury than MI alone in terms of the morphological or hemodynamic parameters. Vascular reactivity increased in all groups, but the combination of HgCl2-MI increased the vasorelaxation induced by ACh compared with the HgCl2 and MI groups. Results showed reduced endothelial nitric oxide synthase (eNOS) protein expression in the MI group; increased iNOS activity in the HgCl2-MI group, although without enough magnitude to reverse the reduction in NO bioavailability; and increased phenylephrine response in the HgCl2-MI group due to an increase in ROS production, notably via xanthine oxidase (XO). Results suggest that the combination of 1 month pre-exposure of HgCl2 before MI changed the endothelial generation of oxidative stress induced by mercury exposure from NADPH oxidase pathway to XO (xanthine oxidase)-dependent ROS production.







Similar content being viewed by others
References
Clarkson, T. W., Magos, L., & Myers, G. J. (2003). The toxicology of mercury—current exposures and clinical manifestations. The New England Journal of Medicine, 349, 1731–1737.
Langworth, S., Sällsten, G., Barregard, L., Cynkier, I., Lind, M. L., & Söderman, E. (1997). Exposure to mercury vapour and impact on health in the dental profession in Sweden. Journal of Dental Research, 76, 1397–1404.
McKelvey, W., Gwynn, R. C., Jeffery, N., Kass, D., Thorpe, L. E., Garg, R. K., et al. (2007). A biomonitoring study of lead, cadmium, and mercury in the blood of New York city adults. Environmental Health Perspectives, 115, 1435–1441.
Salonen, J. T., Seppänen, K., Nyyssönen, K., Korpela, H., Kauhanen, J., Kantola, M., et al. (1995). Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation, 91, 645–655.
Virtanen, J. K., Voutilainen, S., Rissanen, T. H., Mursu, J., Tuomainen, T. P., Korhonen, M. J., et al. (2005). Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arteriosclerosis, Thrombosis, and Vascular Biology, 25, 228–233.
Choi, A. L., Weihe, P., Budtz-Jørgensen, E., Jørgensen, P. J., Salonen, J. T., Tuomainen, T. P., et al. (2009). Methylmercury exposure and adverse cardiovascular effects in faroese whaling men. Environmental Health Perspectives, 117(3), 367–372.
Vassallo, D. V., Simões, M. R., Furieri, L. B., Fioresi, M., Fiorim, J., Almeida, E. A., et al. (2011). Toxic effects of mercury, lead and gadolinium on vascular reactivity. Brazilian Journal of Medical and Biological Research, 44, 939–946.
Lemos, N. B., Angeli, J. K., Faria, T. de O., Ribeiro Junior, R. F., Vassallo, D. V., Padilha, A. S., et al. (2012). Low mercury concentration produces vasoconstriction, decreases nitric oxide bioavailability and increases oxidative stress in rat conductance artery. PLoS ONE, 7(11), e49005.
Rajendran, P., Rengarajan, T., Thangavel, J., Nishigaki, Y., Sakthisekaran, D., Sethi, G., et al. (2013). The vascular endothelium and human diseases. International Journal of Biological Sciences, 9, 1057–1069.
Guallar, E., Sanz-Gallardo, M. I., van’t Veer, P., Bode, O., Aro, A., Gómez-Aracena, J., et al. (2002). Mercury, fish oils, and the risk of myocardial infarction. The New England Journal of Medicine, 347, 1747–1754.
Houston, M. C. (2007). The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Alternative Therapies in Health and Medicine, 13, S128–S133.
Houston, M. C. (2011). Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. Journal of Clinical Hypertension (Greenwich), 13, 621–627.
Park, S. K., Lee, S., Basu, N., & Franzblau, A. (2013). Associations of blood and urinary mercury with hypertension in U.S. adults: The NHANES 2003–2006. Environmental Research, 123, 25–32.
Furieri, L. B., Galán, M., Avendaño, M. S., García-Redondo, A. B., Aguado, A., Martínez, S., et al. (2011). Endothelial dysfunction of rat coronary arteries after exposure to low concentrations of mercury is dependent on reactive oxygen species. British Journal of Pharmacology, 162, 1819–1831.
Peçanha, F. M., Wiggers, G. A., Briones, A. M., Perez-Giron, J. V., Miguel, M., Garcia-Redondo, A. B., et al. (2010). The role of cyclooxygenase (COX)-2 derived prostanoids on vasoconstrictor responses to phenylephrine is increased by exposure to mercury concentration. Journal of Physiology and Pharmacology, l61(1), 29–36.
Kishimoto, T., Oguri, T., & Tada, M. (1995). Effect of methylmercury (CH3HgCl) injury on nitric oxide synthase (NOS) activity in cultured human umbilical vascular endothelial cells. Toxicology, 103, 1–7.
Salonen, J. T., Seppanen, K., Lakka, T. A., Salonen, R., & Kaplan, G. A. (2000). Mercury accumulation and accelerated progression of carotid atherosclerosis: a population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis, 148, 265–273.
Harja, E., Bu, D. X., Hudson, B. I., Chang, J. S., Shen, X., Hallam, K., et al. (2008). Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE −/− mice. The Journal of Clinical Investigation, 118, 183–194.
Go, A. S., Mozaffarian, D., Roger, V. L., Benjamin, E. J., Berry, J. D., Borden, W. B., et al. (2013). Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation, 127, e6–e245.
Faria, T. de O., Baldo, M. P., Simões, M. R., Pereira, R. B., Mill, J. G., Vassallo, D. V., et al. (2011). Body weight loss after myocardial infarction in rats as a marker of early heart failure development. Archives of Medical Research, 42, 274–280.
Mill, J. G., Stefanon, I., dos Santos, L., & Baldo, M. P. (2011). Remodeling in the ischemic heart: the stepwise progression for heart failure. Brazilian Journal of Medical and Biological Research, 44, 890–898.
Stefanon, I., Valero-Muñoz, M., Fernandes, A. A., Ribeiro, R. F., Jr., Rodríguez, C., Miana, M., et al. (2013). Left and right ventricle late remodeling following myocardial infarction in rats. PLoS ONE, 8, e64986.
Bauersachs, J., & Widder, J. D. (2008). Endothelial dysfunction in heart failure. Pharmacological Reports, 60, 119–126.
Förstermann, U. (2008). Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nature Clinical Practice Cardiovascular Medicine, 5, 338–349.
Weseler, A. R., & Bast, A. (2010). Oxidative stress and vascular function: implications for pharmacologic treatment. Current Hypertension Reports, 12, 154–161.
Abbate, A., Santini, D., Biondi-Zoccai, G. G. L., Scarpa, S., Vasaturo, F., Liuzzo, G., et al. (2004). Cyclo-oxygenase-2 (COX-2) expression at the site of recent myocardial infarction: friend or foe? Heart, 90(4), 440–443.
Looi, Y. H., Grieve, D. J., Siva, A., Walker, S. J., Anilkumar, N., Cave, A. C., et al. (2008). Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension, 51(2), 319–325.
Landmesser, U., Spiekermann, S., Dikalov, S., Tatge, H., Wilke, R., et al. (2002). Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation, 106, 3073–3078.
Engberding, N., Spiekermann, S., Schaefer, A., Heineke, A., Wiencke, A., Müller, M., et al. (2004). Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug. Circulation, 110, 2175–2179.
Zweier, J. L., & Talukder, M. A. (2006). The role of oxidants and free radicals in 32 reperfusion injury. Cardiovascular Research, 70(2), 181–190.
Sagor, M. A., Tabassum, N., Potol, M. A., & Alam, M. A. (2015). Xanthine oxidase inhibitor, allopurinol, prevented oxidative stress, fibrosis, and myocardial damage in isoproterenol induced aged rats. Oxidative Medicine and Cellular Longevity, 2015, 478039.
da Cunha, V., Stefanon, I., & Mill, J. G. (2004). The role of nitric oxide in mediating cardiovascular alterations accompanying heart failure in rats. Canadian Journal of Physiology and Pharmacology, 82, 372–379.
Sartório, C. L., Pinto, V. D., Cutini, G. J., Vassallo, D. V., & Stefanon, I. (2005). Effects of inducible nitric oxide syinthase inhibition on the rat tail vascular bed reactivity three days after myocardial infarction. Journal of Cardiovascular Pharmacology, 45, 321–326.
Faria, T. de O., Costa, G. P., Almenara, C. C., Angeli, J. K., Vassallo, D. V., Stefanon, I., et al. (2014). Chronic exposure to low doses of HgCl2 avoids calcium handling impairment in the right ventricle after myocardial infarction in rats. PLoS ONE, 9(4), e95639.
Wiggers, G. A., Peçanha, F. M., Briones, A. M., Pérez-Girón, J. V., Miguel, M., Vassallo, D. V., et al. (2008). Low mercury concentrations cause oxidative stress and endothelial dysfunction in conductance and resistance arteries. American Journal of Physiology Heart and Circulatory Physiology, 295, H1033–H1043.
Gupta, M., Bansal, J. K., & Khanna, C. M. (1996). Blood mercury in workers exposed to the preparation of mercury cadmium telluride layers on cadmium telluride base. Industrial Health, 34, 421–425.
Chen, C., Qu, L., Li, B., Xing, L., Jia, G., Wang, T., et al. (2005). Increased oxidative DNA damage, as assessed by urinary 8-hydroxy-2-deoxyguanosine concentrations, and serum redox status in persons exposed to mercury. Clinical Chemistry, 51, 759–767.
George, J., & Struthers, A. D. (2009). Role of urate, xanthine oxidase and the effects of allopurinol in vascular oxidative stress. Vascular Health and Risk Management, 5, 265–272.
O’Rourke, M. F., & Pauca, A. L. (2004). Augmentation of the aortic and central arterial pressure waveform. Blood Pressure Monitoring, 9, 179–185.
Rizzetti, D. A., Torres, J. G., Escobar, A. G., Peçanha, F. M., Santos, F. W., Puntel, R. L., et al. (2013). Apocynin prevents vascular effects caused by chronic exposure to low concentrations of mercury. PLoS ONE, 8, e55806.
Furieri, L. B., Fioresi, M., Junior, R. F., Bartolomé, M. V., Fernandes, A. A., Cachofeiro, V., et al. (2011). Exposure to low mercury concentration in vivo impairs myocardial contractile function. Toxicology and Applied Pharmacology, 255(2), 193–199. doi:10.1016/j.taap.2011.06.015.
Schramm, A., Matusik, P., Osmenda, G., & Guzik, T. J. (2012). Targeting NADPH oxidases in vascular pharmacology. Vascular Pharmacology, 56, 216–231.
Hare, J. M., & Stamler, J. S. (2005). NO/Redox disequilibrium in the failing heart and cardiovascular system. The Journal of Clinical Investigation, 115, 509–517.
Ahn, B., Beharry, A. W., Frye, G. S., Judge, A. R., & Ferreira, L. F. (2015). NAD(P)H oxidase subunit p47phox knockout prevents diaphragm contractile dysfunction in heart failures. American Journal of Physiology. Lung Cellular and Molecular Physiology, 309, L497–L505.
Xiao, J., She, Q., Wang, Y., Luo, K., Yin, Y., Hu, R., et al. (2009). Effect of allopurinol on cardiomyocyte apoptopsis in rats after myocardial infarction. European Journal of Heart Failure, 11, 20–27.
Cai, H., & Harrison, D. G. (2000). Endothelial dysfunction in cardiovascular disease: The role of oxidative stress. Circulation Research, 87, 840–844.
Förstermann, U. (2010). Nitric oxide and oxidative stress in vascular disease. Pflügers Archv, 459, 923–939.
Acknowledgements
This study was supported by Grants from No 54685435/2011-FAPES (Fundação de Amparo à Pesquisa do Espírito Santo), No 48511935/2009 PRONEX—FAPES/CNPq (FAPES/Conselho Nacional de Desenvolvimento Cientifico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faria, T.d., Simões, M.R., Vassallo, D.V. et al. Xanthine Oxidase Activation Modulates the Endothelial (Vascular) Dysfunction Related to HgCl2 Exposure Plus Myocardial Infarction in Rats. Cardiovasc Toxicol 18, 161–174 (2018). https://doi.org/10.1007/s12012-017-9427-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-017-9427-x