Abstract
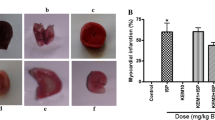
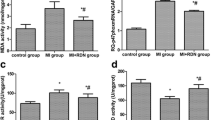
Several researchers studied the protective effect of the N-acetylcysteine (NAC) when it was given before the induction of myocardial infarction (MI). Other researchers studied such protective effect when it was before done and after done of the MI. The missing data are the comparison between the protective effect of NAC before myocardial injury with its protective effect both before and after myocardial injury. The aim of the study was to compare the cardioprotective effect of NAC on the isoprenaline-induced myocardial injury before the isoprenaline (ISP) injection with its protective effect both before and after the ISP injection. This study was applied over both short and long time periods. A total of 90 male adult Wistar albino rats were used in the study. The rats were divided into four groups: control group, ISP-treated group, NAC-pretreated group and NAC-pre-& posttreated group. Based on the duration of the experiment, the second and third groups were further subdivided into a and b groups. Histological, immunohistochemical and histomorphometric analysis were used. The myocytes in the ISP-treated groups were fragmented, disrupted with karyolysis. The blood vessels were dilated, congested and associated with blood extravasation, interstitial edema and cellular inflammatory infiltration. Much improvement was observed in the NAC-pretreated group. Focal degeneration was detected in the muscle fibers. The capillaries were normal. Minimal blood extravasation and cellular infiltration were seen. The cardiac muscle fibers in the NAC-pre-& posttreated group were regularly arranged. The mean collagen fiber area percent of the ISP-treated groups was significantly higher by 8.3-folds and 10.1-folds as compared with that of the control group and was also higher by 5.5-folds and 6.8-folds as compared with that of the NAC-pre-&posttreated groups. The α-SMA area percent in the ISP-treated groups was significantly higher by 12.2-folds and 23.9-folds as compared with that of the control group and was higher by 7.5-folds and 15-folds as compared with that of the NAC-pre-& posttreated groups. The mean PCNA area percent of the ISP-treated groups was significantly higher by 126.2 and 164.8% as compared with that of the control group and was higher by 106.3 and 141.5% as compared with that of NAC-pre-& posttreated groups. ISP had deleterious effects on the heart. Administration of NAC before ISP injection could largely reduce the ISP-induced short- and long-term alterations. The protection was maximum with the use of NAC before the ISP injection and continued after the injection for 12 days.










Similar content being viewed by others
References
Thygesen, K., et al. (2007). Universal definition of myocardial infarction. European Heart Journal, 28(20), 2525–2538.
Pereira, C. A., et al. (2013). Anemia, heart failure and evidence-based clinical management. Arquivos Brasileiros de Cardiologia, 101(1), 87–92.
Almahmeed, W., et al. (2012). Coronary artery disease in Africa and the Middle East. Therapeutics and Clinical Risk Management, 8, 65–72.
Mozaffarian, D., et al. (2016). Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation, 133(4), e38–e360.
Brouri, F., et al. (2002). Toxic cardiac effects of catecholamines: Role of beta-adrenoceptor downregulation. European Journal of Pharmacology, 456(1–3), 69–75.
Gayathri, K., et al. (2011). Cardioprotective effect of lemon grass as evidenced by biochemical and histopathological changes in experimentally induced cardiotoxicity. Human and Experimental Toxicology, 30(8), 1073–1082.
Millea, P. J. (2009). N-acetylcysteine: Multiple clinical applications. American Family Physician, 80(3), 265–269.
Abe, M., et al. (2008). Comparison of the protective effect of N-acetylcysteine by different treatments on rat myocardial ischemia-reperfusion injury. Journal of Pharmacological Sciences, 106(4), 571–577.
Wu, X. Y., et al. (2014). N-acetylcysteine reduces oxidative stress, nuclear factor-κB activity and cardiomyocyte apoptosis in heart failure. Molecular Medicine Reports, 10(2), 615–624.
Winniford, M. D., et al. (1986). Potentiation of nitroglycerin-induced coronary dilatation by N-acetylcysteine. Circulation, 73(1), 138–142.
Meeran, N., Fizur, M., Prince, M., & Stanely, P. (2011). Protective effects of N-acetyl cysteine on lipid peroxide metabolism on isoproterenol-induced myocardial infarcted rats. Journal of Biochemical and Molecular Toxicology, 25(3), 151–157.
Liu, B., et al. (2009). Protective effects of N-acetylcysteine in isoproterenol-induced myocardium injury in rats. Molecular Biology Reports, 36(4), 761–765.
Haleagrahara, N., Julian, V., & Chakravarthi, S. (2011). N-acetylcysteine offers cardioprotection by decreasing cardiac lipid hydroperoxides and 8-isoprostane level in isoproterenol-induced cardiotoxicity in rats. Cardiovascular Toxicology, 11(4), 373–381.
Upaganlawar, A., Gandhi, C., & Balaraman, R. (2009). Effect of green tea and vitamin E combination in isoproterenol induced myocardial infarction in rats. Plant Foods for Human Nutrition, 64(1), 75–80.
Malliaras, K., et al. (2013). Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. EMBO Molecular Medicine, 5(2), 191–209.
Neri, M., et al. (2015). Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Current Vascular Pharmacology, 13(1), 26–36.
Forechi, L., et al. (2012). Granulocyte colony-stimulating factor improves early remodeling in isoproterenol-induced cardiac injury in rats. Pharmacological Reports, 64(3), 643–649.
Tanwar, V., et al. (2010). Curcumin protects rat myocardium against isoproterenol-induced ischemic injury: Attenuation of ventricular dysfunction through increased expression of Hsp27 along with strengthening antioxidant defense system. Journal of Cardiovascular Pharmacology, 55(4), 377–384.
Sciarretta, S., et al. (2012). Rheb is a critical regulator of autophagy during myocardial ischemia: Pathophysiological implications in obesity and metabolic syndrome. Circulation, 125(9), 1134–1146.
Wang, X. F., et al. (2013). Ginsenoside rb1 reduces isoproterenol-induced cardiomyocytes apoptosis in vitro and in vivo. Evidence-Based Complementary and Alternative Medicine, 2013, 454389.
Zhuo, X. Z., et al. (2013). Isoproterenol instigates cardiomyocyte apoptosis and heart failure via AMPK inactivation-mediated endoplasmic reticulum stress. Apoptosis, 18(7), 800–810.
Lu, F. H., et al. (2013). Role of the calcium-sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cellular Physiology and Biochemistry, 31(4–5), 728–743.
Kakkar, R., & Lee, R. T. (2010). Intramyocardial fibroblast myocyte communication. Circulation Research, 106(1), 47–57.
Davel, A. P., et al. (2006). Changes in vascular reactivity following administration of isoproterenol for 1 week: A role for endothelial modulation. British Journal of Pharmacology, 148(5), 629–639.
Orhan, G., et al. (2006). Effects of N-acetylcysteine on myocardial ischemia-reperfusion injury in bypass surgery. Heart and Vessels, 21(1), 42–47.
Padma, V. V., Devi, C. S., & Ramkumar, K. M. (2006). Modulatory effect of fish oil on the myocardial antioxidant defense system in isoproterenol-induced myocardial infarction. Journal of Basic and Clinical Physiology and Pharmacology, 17(1), 1–15.
Braunersreuther, V., & Jaquet, V. (2012). Reactive oxygen species in myocardial reperfusion injury: From physiopathology to therapeutic approaches. Current Pharmaceutical Biotechnology, 13(1), 97–114.
Virag, J. I., & Murry, C. E. (2003). Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. American Journal of Pathology, 163(6), 2433–2440.
Ghigliotti, G., et al. (2001). N-acetyl-cysteine reduces neointimal thickening and procoagulant activity after balloon-induced injury in abdominal aortae of New Zealand white rabbits. Thrombosis and Haemostasis, 85(4), 724–729.
Hori, Y., et al. (2011). Spironolactone decreases isoproterenol-induced ventricular fibrosis and matrix metalloproteinase-2 in rats. Biological and Pharmaceutical Bulletin, 34(1), 61–65.
Basha, R. H., & Priscilla, D. H. (2013). An in vivo and in vitro study on the protective effects of N-acetylcysteine on mitochondrial dysfunction in isoproterenol treated myocardial infarcted rats. Experimental and Toxicologic Pathology, 65(1–2), 7–14.
Punithavathi, V. R., Shanmugapriya, K., & Prince, P. S. (2010). Protective effects of rutin on mitochondrial damage in isoproterenol-induced cardiotoxic rats: an in vivo and in vitro study. Cardiovascular Toxicology, 10(3), 181–189.
Yin, W., et al. (2009). Stimulation of kappa-opioid receptor reduces isoprenaline-induced cardiac hypertrophy and fibrosis. European Journal of Pharmacology, 607(1–3), 135–142.
Talasaz, A. H., et al. (2014). Effects of N-acetylcysteine on the cardiac remodeling biomarkers and major adverse events following acute myocardial infarction: A randomized clinical trial. American Journal of Cardiovascular Drugs, 14(1), 51–61.
Frangogiannis, N. G. (2014). The inflammatory response in myocardial injury, repair, and remodelling. Nature Reviews Cardiology, 11(5), 255–265.
Naugle, J. E., et al. (2006). Type VI collagen induces cardiac myofibroblast differentiation: Implications for postinfarction remodeling. American Journal of Physiology Heart and Circulatory Physiology, 290(1), H323–H330.
Frangogiannis, N. G., Michael, L. H., & Entman, M. L. (2000). Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb). Cardiovascular Research, 48(1), 89–100.
Jankowski, M., et al. (2010). Anti-inflammatory effect of oxytocin in rat myocardial infarction. Basic Research in Cardiology, 105(2), 205–218.
Takhtfooladi, M. A., et al. (2014). Effects of N-acetylcysteine on liver remote injury after skeletal muscle ischemia reperfusion in rats. The Turkish Journal of Gastroenterology, 25(Suppl 1), 43–47.
Aitio, M. L. (2006). N-acetylcysteine–passe-partout or much ado about nothing? British Journal of Clinical Pharmacology, 61(1), 5–15.
Lee, T. M., Lai, P. Y., & Chang, N. C. (2010). Effect of N-acetylcysteine on sympathetic hyperinnervation in post-infarcted rat hearts. Cardiovascular Research, 85(1), 137–146.
Wang, C., et al. (2014). N-Acetylcysteine attenuates ischemia-reperfusion-induced apoptosis and autophagy in mouse liver via regulation of the ROS/JNK/Bcl-2 pathway. PLoS ONE, 9(9), e108855.
Sun, Y., et al. (2014). N-acetylcysteine attenuates reactive-oxygen-species-mediated endoplasmic reticulum stress during liver ischemia-reperfusion injury. World Journal of Gastroenterology, 20(41), 15289–15298.
Demiroren, K., et al. (2014). Protective effects of L-carnitine, N-acetylcysteine and genistein in an experimental model of liver fibrosis. Clinics and Research in Hepatology and Gastroenterology, 38(1), 63–72.
Felton, V. M., Borok, Z., & Willis, B. C. (2009). N-Acetylcysteine inhibits alveolar epithelial-mesenchymal transition. American Journal of Physiology. Lung Cellular and Molecular Physiology, 297(5), L805–L812.
Wu, J., et al. (2015). Icariside II inhibits cell proliferation and induces cell cycle arrest through the ROS-p38-p53 signaling pathway in A375 human melanoma cells. Molecular Medicine Reports, 11(1), 410–416.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaki, S.M., Abdalla, I.L., Sadik, A.O.E. et al. Protective Role of N-Acetylcysteine on Isoprenaline-Induced Myocardial Injury: Histological, Immunohistochemical and Morphometric Study. Cardiovasc Toxicol 18, 9–23 (2018). https://doi.org/10.1007/s12012-017-9407-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-017-9407-1