Abstract
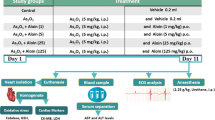
Arsenic trioxide (As2O3) is a highly effective therapeutic against acute promyelocytic leukaemia, but its clinical efficacy is burdened by serious cardiac toxicity. The present study was performed to evaluate the effect of omega (ω)-3 fatty acid on As2O3-induced cardiac toxicity in in vivo and in vitro settings. In in vivo experiments, male Wistar rats were orally administered with As2O3 4 mg/kg body weight for a period of 45 days and cardiotoxicity was assessed. As2O3 significantly increased the tissue arsenic deposition, micronuclei frequency and creatine kinase (CK)-MB activity. There were a rise in lipid peroxidation and a decline in reduced glutathione, glutathione peroxidase, glutathione-S-transferase, superoxide dismutase and catalase in heart tissue of arsenic-administered rats. The cardioprotective role of ω-3 fatty acid was assessed by combination treatment with As2O3. ω-3 fatty acid co-administration with As2O3 significantly alleviated these changes. In in vitro study using H9c2 cardiomyocytes, As2O3 treatment induced alterations in cell viability, lactate dehydrogenase (LDH) release, lipid peroxidation, cellular calcium levels and mitochondrial membrane potential (∆Ψm). ω-3 fatty acid co-treatment significantly increased cardiomyocyte viability, reduced LDH release, lipid peroxidation and intracellular calcium concentration and improved the ∆Ψm. These findings suggested that the ω-3 fatty acid has the potential to protect against As2O3-induced cardiotoxicity.









Similar content being viewed by others
References
IARC. (1987). Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl., 7, 1–440.
Iland, H. J., & Seymour, J. F. (2013). Role of arsenic trioxide in acute promyelocytic leukemia. Current Treatment Options in Oncology, 14(2), 170–184.
Huan, S. Y., Yang, C. H., & Chen, Y. C. (2000). Arsenic trioxide therapy for relapsed acute promyelocytic leukemia: an useful salvage therapy. Leukaemia & Lymphoma, 38(3–4), 283–293.
Barbey, J. T., & Soignet, S. (2001). Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Annals of Internal Medicine, 135, 842–843.
Teng, L. L., Shao, L., Zhao, Y. T., Yu, X., Zhang, D. F., & Zhang, H. (2010). The beneficial effect of n-3 polyunsaturated fatty acids on doxorubicin-induced chronic heart failure in rats. Journal of International Medical Research, 38(3), 940–948.
Stanley, W. C., Khairallah, R. J., & Dabkowski, E. R. (2012). Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care., 15, 122–126.
Leaf, A., Xiao, Y. F., Kang, J. X., & Billman, G. E. (2003). Prevention of sudden cardiac death by n-3 polyunsaturated fatty acids. Pharmacology & Therapeutics, 98, 355–377.
Hardman, W. E. (2002). Omega-3 fatty acids to augment cancer therapy. Journal of Nutrition, 132, 3508s–3512s.
Merendino, N., Costantini, L., Manzi, L., Molinari, R., D’Eliseo, D., & Velotti, F. (2013). Dietary omega-3 polyunsaturated fatty acid DHA: A potential adjuvant in the treatment of cancer. Biomedicine Research International, 2013, 310186.
Hardman, W. E., Avula, C. P., Fernandes, G., & Cameron, I. L. (2001). Three percent dietary fish oil concentrate increased efficacy of doxorubicin against MDA-MB 231 breast cancer xenografts. Clinical Cancer Research, 7(7), 2041–2049.
Mathews, V. V., Paul, M. V., Abhilash, M., Manju, A., Abhilash, S., & Nair, R. H. (2013). Myocardial toxicity of acute promyelocytic leukaemia drug-arsenic trioxide. European Review for Medical and Pharmacological Sciences, 1, 34–38.
Fenech, M. (1993). The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutation Research, 285, 35–44.
Buege, J. A., & Aust, S. D. (1978). The thiobarbituric acid assay. Methods in Enzymology, 52, 306–307.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82(1), 70–77.
Rotruck, J. T., Pope, A. L., Ganther, H. E., Swanson, A. B., Hafeman, D. G., & Hoekstra, W. G. (1973). Selenium: Biochemical role as a component of glutathione peroxidase. Science, 179, 588–590.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249(22), 7130–7139.
Kakkar, P., Das, B., & Viswanathan, P. N. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian Journal of Biochemistry Biophysics, 21(2), 130–132.
Aebi, H. (1974). Catalase. In H. U. Bergmeyer (Ed.), Methods of enzymatic analysis (Vol. 2, pp. 673–678). New York: Academic Press.
Zhao, X. Y., Li, G. Y., Liu, Y., Chai, L. M., Chen, J. X., Zhang, Y., et al. (2008). Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. British Journal of Pharmacology, 154(1), 105–113.
Ratnaike, R. N. (2003). Acute and chronic arsenic toxicity. Postgraduate Medical Journal, 79(933), 391–396.
Li, Y. M., & Broome, J. D. (1999). Arsenic targets tubulins to induce apoptosis in myeloid leukemia cells. Cancer Research, 59, 776–780.
Mathews, V. V., Paul, M. S., Abhilash, M., Manju, A., Abhilash, S., & Nair, R. H. (2014). Mitigation of hepatotoxic effects of arsenic trioxide through omega-3 fatty acid in rats. Toxicology and Industrial Health, 30(9), 806–813.
Bhattacharya, A., Lawrence, R. A., Krishnan, A., Zaman, K., Sun, D., & Fernandes, G. (2003). Effect of dietary n-3 and n-6 oils with and without food restriction on activity of antioxidant enzymes and lipid peroxidation in livers of cyclophosphamide treated autoimmune-prone NZB/W female mice. Journal of the American College of Nutrition, 22(5), 388–399.
Yeh, J. Y., Cheng, L. C., Ou, B. R., Whanger, D. P., & Chang, L. W. (2002). Differential influences of various arsenic compounds on glutathione redox status and antioxidative enzymes in porcine endothelial cells. Cellular and Molecular Life Sciences, 59(11), 1972–1982.
Flora, S. J. (2011). Arsenic-induced oxidative stress and its reversibility. Free Radical Biology and Medicine, 51(2), 257–281.
Li, M., Zhu, Q., Hu, C., Giesy, J. P., Kong, Z., & Cui, Y. (2011). Protective effects of eicosapentaenoic acid on genotoxicity and oxidative stress of cyclophosphamide in mice. Environmental Toxicology, 26(3), 217–223.
Thompson, J. A., White, C. C., Cox, D. P., Chan, J. Y., Kavanagh, T. J., Fausto, N., & Franklin, C. C. (2009). Distinct Nrf1/2-independent mechanisms mediate As 3+-induced glutamatecysteine ligase subunit gene expression in murine hepatocytes. Free Radical Biology and Medicine, 46, 1614–1625.
Komatsu, W., Ishihara, K., Murata, M., Saito, H., & Shinohara, K. (2003). Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-gamma plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radical Biology and Medicine, 34(8), 1006–1016.
Kumar, S. H. S., & Anandan, R. (2007). Biochemical studies on the cardioprotective effect of glutamine on tissue antioxidant defense system in isoprenaline-induced myocardial infarction in rats. Journal of Clinical Biochemistry and Nutrition, 40(1), 49–55.
Hays, A. M., Lantz, R. C., Rodgers, L. S., Sollome, J. J., Vaillancourt, R. R., Andrew, A. S., et al. (2008). Arsenic-induced decreases in the vascular matrix. Toxicologic Pathology, 36, 805–817.
Iraz, M., Erdogan, H., Ozyurt, B., Ozugurlu, F., Ozgocmen, S., & Fadillioglu, E. (2005). Brief communication: omega-3 essential fatty acid supplementation and erythrocyte oxidant/antioxidant status in rats. Annals of Clinical and Laboratory Science, 35(2), 169–173.
World Health Organization (2015) Interim summary of conclusions and dietary recommendations on total fat. http://www.who.int/nutrition/topics/FFA_summary_rec_conclusion.pdf. Accessed December 18, 2015.
American Heart Association, “Fish 101,” (2014).http://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/Fish-101_UCM_305986_Article.jsp. Accessed December 18, 2015.
Fappi, A., Godoy, T. S., Maximino, J. R., Rizzato, V. R., de Neves, J. C., Chadi, G., & Zanoteli, E. (2014). The effects of omega-3 fatty acid supplementation on dexamethasone-induced muscle atrophy. Biomedicine Research International, 2014, 961438.
Zhao, X., Feng, T., Chen, H., Shan, H., Zhang, Y., Lu, Y., & Yang, B. (2008). Arsenic trioxide-induced apoptosis in H9c2 cardiomyocytes: implications in cardiotoxicity. Basic & Clinical Pharmacology & Toxicology, 102(5), 419–425.
Raghu, K. G., & Cherian, O. L. (2009). Characterization of cytotoxicity induced by arsenic trioxide (a potent anti-APL drug) in rat cardiac myocytes. Journal of Trace Elements in Medicine and Biology, 23, 61–68.
Crow, M. T., Mani, K., Nam, Y. J., & Kitsis, R. N. (2004). The mitochondrial death pathway and cardiac myocyte apoptosis. Circulation Research, 95, 957–970.
Halestrap, A. P. (2009). What is the mitochondrial permeability transition pore? Journal of Molecular and Cellular Cardiology, 46, 821–831.
O’Shea, K. M., Khairallah, R. J., Sparagna, G. C., Xu, W., Hecker, P. A., Robillard-Frayne, I., et al. (2009). Dietary omega-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. Journal of Molecular and Cellular Cardiology, 47(6), 819–827.
Khairallah, R. J., Sparagna, G. C., Khanna, N., O’Shea, K. M., Hecker, P. A., Kristian, T., et al. (2010). Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition. Biochimica et Biophysica Acta, 1797(8), 1555–1562.
Acknowledgments
This work was supported by University Grants Commission, New Delhi (F. No.: 39-683/2010SR), and awarded the research fellowship in sciences for meritorious student to Mr. Mathews V. Varghese (F. No.: 4-1/2006 (BSR)/11-29/2008(BSR)). We are grateful to Prof. C.C Kartha, Professor of Eminence, Cardiovascular Disease Biology, Rajiv Gandhi Centre for Biotechnology, Trivandrum, for providing laboratory facilities during in vitro studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Varghese, M.V., Abhilash, M., Paul, M.V.S. et al. Omega-3 Fatty Acid Protects Against Arsenic Trioxide-Induced Cardiotoxicity In Vitro and In Vivo. Cardiovasc Toxicol 17, 109–119 (2017). https://doi.org/10.1007/s12012-016-9361-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-016-9361-3