Abstract
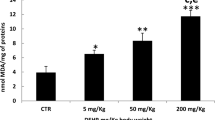
Preventive effects of ellagic acid against doxorubicin-induced cardiac oxidative, inflammatory and apoptotic stress were examined. This agent at 0.25, 0.5 or 1 % was added in feed and supplied to mice for 8 weeks, and followed by doxorubicin treatment. Ellagic acid intake increased its deposit in heart. Pre-intake of this compound at 0.5 and 1 % significantly attenuated doxorubicin caused increase in plasma creatine phosphokinase activity. Doxorubicin treatment decreased glutathione content, increased reactive oxygen species (ROS), malonyldialdehyde (MDA), interleukin (IL)-6, IL-10, monocyte chemoattractant protein-1 and tumor necrosis factor-alpha levels, declined glutathione peroxidase (GPX) and superoxide dismutase (SOD) activities, and enhanced xanthine oxidases (XO) activity in heart. Ellagic acid intake dose-dependently reserved glutathione content, lowered ROS and MDA levels, and reduced XO activity. This compound at 0.5 and 1 % retained GPX and SOD activities, and decreased cytokines in heart. Doxorubicin treatment raised cardiac activity and protein production of caspase-3, nuclear factor kappa B (NF-κB) p50 and p65. Ellagic acid dose-dependently lowered caspase-3 activity and cleaved caspase-3 formation, and at 0.5 and 1 % declined activity and protein level of NF-κB. Doxorubicin treatment also up-regulated cardiac expression of p-p38, p-ERK 1/2 and p-JNK, and ellagic acid at 0.5 and 1 % suppressed p-p38 expression and at 1 % down-regulated p-ERK 1/2 expression. These findings suggest that ellagic acid is a potent cardiac protective agent against doxorubicin.




Similar content being viewed by others
Abbreviations
- AT:
-
Antithrombin
- CPK:
-
Creatine phosphokinase
- CRP:
-
c-Reactive protein
- GPX:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- IL:
-
Interleukin
- LDH:
-
Lactate dehydrogenase
- MAPK:
-
Mitogen-activated protein kinase
- MCP:
-
Monocyte chemoattractant protein
- MDA:
-
Malonyldialdehyde
- NF-κB:
-
Nuclear factor kappa B
- PAI:
-
Plasminogen activator inhibitor
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TNF:
-
Tumor necrosis factor
- XO:
-
Xanthine oxidases
References
Frei, B. L., & Soefje, S. A. E. (2008). A review of the cardiovascular effects of oncology agents. Journal of Pharmacy Practice, 21, 146–158.
Carvalho, C., Santos, R. X., Cardoso, S., Correia, S., Oliveira, P. J., Santos, M. S., et al. (2009). Doxorubicin: The good, the bad and the ugly effect. Current Medicinal Chemistry, 16, 3267–3285.
Huh, W. W., Jaffe, N., Durand, J. B., Munsell, M. F., & Herzog, C. E. (2010). Comparison of doxorubicin cardiotoxicity in pediatric sarcoma patients when given with dexrazoxane versus as continuous infusion. Pediatric Hematology and Oncology, 27, 546–557.
Octavia, Y., Tocchetti, C. G., Gabrielson, K. L., Janssens, S., Crijns, H. J., & Moens, A. L. (2012). Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. Journal of Molecular and Cell Cardiology, 52, 1213–1225.
Kalivendi, S. V., Konorev, E. A., Cunningham, S., Vanamala, S. K., Kaji, E. H., Joseph, J., et al. (2005). Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: Role of mitochondrial reactive oxygen species and calcium. Biochemistry Journal, 389, 527–539.
Li, S. E. M., & Yu, B. (2008). Adriamycin induces myocardium apoptosis through activation of nuclear factor kappaB in rat. Molecular Biology Reports, 35, 489–494.
Kang, Y. J., Zhou, Z. X., Wang, G. W., Buridi, A., & Klein, J. B. (2000). Suppression by metallothionein of doxorubicin-induced cardiomyocyte apoptosis through inhibition of p38 mitogen-activated protein kinases. Journal of Biological Chemistry, 275, 13690–13698.
Ueno, M., Kakinuma, Y., Yuhki, K., Murakoshi, N., Iemitsu, M., Miyauchi, T., et al. (2006). Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. Journal of Pharmacological Science, 101, 151–158.
Lou, H., Danelisen, I., & Singal, P. K. (2005). Involvement of mitogen-activated protein kinases in adriamycin-induced cardiomyopathy. American Journal of Physiology-Heart and Circulatory Physiology, 288, H1925–H1930.
Swystun, L. L., Mukherjee, S., & Liaw, P. C. (2011). Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. Journal of Thrombosis and Haemostasis, 9, 2313–2321.
Sellappan, S., Akoh, C. C., & Krewer, G. (2002). Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. Journal of Agricultural and Food Chemistry, 50, 2432–2438.
Makena, P. S., & Chung, K. T. (2007). Effects of various plant polyphenols on bladder carcinogen benzidine-induced mutagenicity. Food and Chemical Toxicology, 45, 1899–1909.
Prakash, D., Suri, S., Upadhyay, G., & Singh, B. N. (2007). Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. International Journal of Food Science and Nutrition, 58, 18–28.
Rosillo, M. A., Sánchez-Hidalgo, M., Cárdeno, A., Aparicio-Soto, M., Sánchez-Fidalgo, S., Villegas, I., et al. (2012). Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacology Research, 66, 235–242.
Devipriya, N., Sudheer, A. R., Srinivasan, M., & Menon, V. P. (2007). Effect of ellagic acid, a plant polyphenol, on fibrotic markers (MMPs and TIMPs) during alcohol-induced hepatotoxicity. Toxicology Mechanisms and Methods, 17, 349–356.
Chao, P. C., Hsu, C. C., & Yin, M. C. (2009). Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutrition & Metabolism, 6, 33.
Kannan, M. M., & Quine, S. D. (2011). Ellagic acid ameliorates isoproterenol induced oxidative stress: Evidence from electrocardiological, biochemical and histological study. European Journal of Pharmacology, 659, 45–52.
Yamada, Y., Yasui, H., & Sakurai, H. (2006). Suppressive effect of caffeic acid and its derivatives on the generation of UVA-induced reactive oxygen species in the skin of hairless mice and pharmacokinetic analysis on organ distribution of caffeic acid in ddY mice. Photochemistry and Photobiology, 82, 1668–1676.
Privratsky, J. R., Wold, L. E., Sowers, J. R., Quinn, M. T., & Ren, J. (2003). AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension, 42, 206–212.
Yamamoto, Y., Hoshino, Y., Ito, T., Nariai, T., Mohri, T., Obana, M., et al. (2008). Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovascular Research, 79, 89–96.
Trivedi, P. P., Kushwaha, S., Tripathi, D. N., & Jena, G. B. (2011). Cardioprotective effects of hesperetin against doxorubicin-induced oxidative stress and DNA damage in rat. Cardiovascular Toxicology, 11, 215–225.
Chen, Y. L., Loh, S. H., Chen, J. J., & Tsai, C. S. (2012). Urotensin II prevents cardiomyocyte apoptosis induced by doxorubicin via Akt and ERK. European Journal of Pharmacology, 680, 88–94.
Pointon, A. V., Walker, T. M., Phillips, K. M., Luo, J., Riley, J., Zhang, S. D., et al. (2010). Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. PLoS ONE, 5, e12733.
Doehner, W., & Landmesser, U. (2011). Xanthine oxidase and uric acid in cardiovascular disease: clinical impact and therapeutic options. Seminars in Nephrology, 31, 433–440.
Szasz, T., Thompson, J. M., & Watts, S. W. (2008). A comparison of reactive oxygen species metabolism in the rat aorta and vena cava: Focus on xanthine oxidase. American Journal of Physiology-Heart and Circulatory Physiology, 295, H1341–H1350.
Morris, P. G., Chen, C., Steingart, R., Fleisher, M., Lin, N., Moy, B., et al. (2011). Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clinical Cancer Research, 17, 3490–3499.
Teng, L. L., Shao, L., Zhao, Y. T., Yu, X., Zhang, D. F., & Zhang, H. (2010). The beneficial effect of n-3 polyunsaturated fatty acids on doxorubicin-induced chronic heart failure in rats. Journal of International Medical Research, 38, 940–948.
Martinovic, I., Abegunewardene, N., Seul, M., Vosseler, M., Horstick, G., Buerke, M., et al. (2005). Elevated monocyte chemoattractant protein-1 serum levels in patients at risk for coronary artery disease. Circulation Journal, 69, 1484–1489.
Das, J., Ghosh, J., Manna, P., & Sil, P. C. (2011). Taurine suppresses doxorubicin-triggered oxidative stress and cardiac apoptosis in rat via up-regulation of PI3-K/Akt and inhibition of p53, p38-JNK. Biochemical Pharmacology, 81, 891–909.
Kim, S. H., Lim, K. M., Noh, J. Y., Kim, K., Kang, S., Chang, Y. K., et al. (2011). Doxorubicin-induced platelet procoagulant activities: An important clue for chemotherapy-associated thrombosis. Toxicology Science, 124, 215–224.
Chen, B., Tuuli, M. G., Longtine, M. S., Shin, J. S., Lawrence, R., Inder, T., et al. (2012). Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. American Journal of Physiology-Endocrinology and Metabolism, 302, E1142–E1152.
Abe, L. T., Lajolo, F. M., & Genovese, M. I. (2012). Potential dietary sources of ellagic acid and other antioxidants among fruits consumed in Brazil: jabuticaba (Myrciaria jaboticaba (Vell.) Berg). Journal of the Science of Food and Agriculture, 92, 1679–1687.
Wang, N., Wang, Z. Y., Mo, S. L., Loo, T. Y., Wang, D. M., & Luo, H. B. (2012). Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via VEGFR-2 signaling pathway in breast cancer. Breast Cancer Research and Treatment, 134, 943–955.
Li, T. M., Chen, G. W., Su, C. C., Lin, J. G., Yeh, C. C., Cheng, K. C., et al. (2005). Ellagic acid induced p53/p21 expression, G1 arrest and apoptosis in human bladder cancer T24 cells. Anticancer Research, 25, 971–979.
Falsaperla, M., Morgia, G., Tartarone, A., Ardito, R., & Romano, G. (2005). Support ellagic acid therapy in patients with hormone refractory prostate cancer (HRPC) on standard chemotherapy using vinorelbine and estramustine phosphate. European Urology, 47, 449–454.
Ertam, I., Mutlu, B., Unal, I., Alper, S., Kivçak, B., & Ozer, O. (2008). Efficiency of ellagic acid and arbutin in melasma: A randomized, prospective, open-label study. Journal of Dermatology, 35, 570–574.
Dorai, T., & Aggarwal, B. B. (2004). Role of chemopreventive agents in cancer therapy. Cancer Letter, 215, 129–140.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, Mc., Yin, Mc. Preventive Effects of Ellagic Acid Against Doxorubicin-Induced Cardio-Toxicity in Mice. Cardiovasc Toxicol 13, 185–193 (2013). https://doi.org/10.1007/s12012-013-9197-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-013-9197-z