Abstract
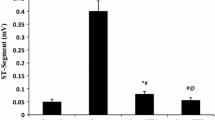
The present study was designed to investigate the cardioprotective effect of neferine against isoproterenol-induced myocardial infarction. Neferine was given orally for 30 days, and isoproterenol was injected subcutaneously for 2 days. Histopathological examination of heart tissue of isoproterenol-treated rats showed myocardial necrosis. Biochemical analysis of isoproterenol-treated rats showed significant increase in the serum marker enzymes—creatine kinase, lactate dehydrogenase, and aspartate transaminase and increased serum glycoprotein components with a concomitant decrease in the heart tissue homogenate when compared to control. Increased lipid peroxidation and decreased antioxidants reduced glutathione, superoxide dismutase, catalase, glutathione-S-transferase, glutathione peroxidase and altered lipid profile in serum and tissue was also recorded in the isoproterenol-treated rats, whereas the rats which received neferine pre-treatment followed by isoproterenol injection showed minimal histological changes, absence of inflammation, and a significant decrease in the serum marker enzymes and serum glycoprotein components with a concomitant increase in the heart tissue homogenate when compared to isoproterenol group. Neferine pre-treatment restored the altered biochemical parameters and lipid profile to near normal. The results of the present study showed that neferine exerts strong antioxidant property against isoproterenol-induced oxidative stress and can be used as a potent cardioprotective agent against isoproterenol-induced myocardial infarction.





Similar content being viewed by others
Abbreviations
- CVD:
-
Cardiovascular diseases
- MI:
-
Myocardial infarction
- MN:
-
Myocardial necrosis
- CK:
-
Creatine kinase
- LDH:
-
Lactate dehydrogenase
- AST:
-
Aspartate transaminase
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- VLDL:
-
Very low-density lipoprotein
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GSH:
-
Reduced glutathione
- GST:
-
Glutathione-S-transferase
- GPx:
-
Glutathione peroxidase
References
Agarwal, V. K., Basannan, D. R., Sinh, R. P., Dutt, M., Abraham, D., & Mustafa, M. S. (2006). Coronary risk factors in a rural community. Indian Journal of Public Health, 50, 19–23.
Smith, S. C., Jackson, R., Pearson, T. A., Fuster, V., Yusuf, S., Faergeman, O., et al. (2004). Principles for national and regional guidelines on cardiovascular disease prevention. Circulation, 109, 3112–3121.
Sushamakumari, S., Jayadeep, A., Kumar, J. S., & Menon, V. P. (1989). Effect of carnitine on malondialdehyde, taurine and glutathione levels in heart of rats subjected to myocardial stress by isoproterenol. Indian Journal of Experimental Biology, 27, 134–137.
Nirmala, C., & Puvanakrishnan, R. (1996). Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Molecular and Cellular Biochemistry, 159, 85–93.
De Biase, L., Pignatelli, P., Lenti, L., Tocci, G., Piccioni, F., Riondino, S., et al. (2003). Enhanced TNF alpha and oxidative stress in patients with heart failure: Effect of TNF alpha on platelet O2-production. Thrombosis and Haemostasis, 90, 317–325.
Rajadurai, M., & Stanely Mainzen Prince, P. (2007). Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats. Toxicology, 230, 178–188.
Rajadurai, M., & Stanely Mainzen Prince, P. (2007). Preventive effect of naringin on isoproterenol-induced cardiotoxicity in Wistar rats: An in vivo and in vitro study. Toxicology, 232, 216–225.
Chatelain, P., Gremel, M., & Brotelle, R. (1987). Prevention by amiodarone of phospholipid depletion in isoproterenol-induced ischemia in rats. European Journal of Pharmacology, 144, 83–90.
Wexler, B. C. (1978). Myocardial infarction in young vs old male rats: Pathophysiologic changes. American Heart Journal, 96, 70–80.
Rathore, N., John, S., Kale, M., & Bhatnagar, D. (1998). Lipid peroxidation and antioxidant enzymes in isoproterenol induced oxidative stress in rat tissues. Pharmacological Research, 38, 297–303.
Pauletti, P. M., Castro-Gamboa, I., Siqueira Silva, D. H., Young, M. C. M., Tomazela, D. M., Eberlin, M. N., et al. (2003). New antioxidant C-glucosylxanthones from the stems of Arrabidaea samydoides. Journal of Natural Products, 66, 1384–1387.
Sridhar, K. R., & Bhat, R. (2007). Lotus—a potential nutraceutical source. Journal of Agricultural Technology, 3, 143–155.
Tomita, M., Watanabe, Y., & Furukawa, H. (1961). On the alkaloids of Nelumbo nucifera Gaertn. IV. Isolation of dl-armepavine. Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan, 81, 1644–1647.
Furukawa, T. (1965). Use of diuretics based on their mechanism of action. 9. Effect of diuretics on the renal regulation of water balance and its application to the treatment of diabetes insipidus. Saishin igaku. Modern Medicine, 20, 2609–2621.
Liu, S., Wang, B., Li, X. Z., Qi, L. F., & Liang, Y. Z. (2009). Preparative separation and purification of liensinine, isoliensinine and neferine from seed embryo of Nelumbo nucifera GAERTN using high-speed counter-current chromatography. Journal of Separation Science, 32, 2476–2481.
Gu, D., Li, D., Qi, Z., Shi, S., Hu, M., Liu, D., et al. (2009). Blockade of HERG K+ channel by isoquinoline alkaloid neferine in the stable transfected HEK293 cells. Naunyn-Schmiedeberg’s Archives of Pharmacology, 380, 143–151.
Chen, Y., Fan, G., Wu, H., Wu, Y., & Mitchell, A. (2007). Separation, identification and rapid determination of liensine, isoliensinine and neferine from embryo of the seed of Nelumbo nucifera GAERTN. By liquid chromatography coupled to diode array detector and tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 43, 99–104.
Chen, J., Liu, J.-H., Wang, T., Xiao, H.-J., Yin, C.-P., & Yang, J. (2008). Effects of plant extract neferine on cyclic adenosine monophosphate and cyclic guanosine monophosphate levels in rabbit corpus cavernosum in vitro. Asian Journal of Andrology, 10, 307–312.
Pan, Y., Cai, B., Wang, K., Wang, S., Zhou, S., Yu, X., et al. (2009). Neferine enhances insulin sensitivity in insulin resistant rats. Journal of Ethnopharmacology, 124, 98–102.
Xiong, Y. Q., & Zeng, F. D. (2003). Effect of neferine on toxicodynamics of dichlorvos for inhibiting rabbit cholinesterase. Acta Pharmacologica Sinica, 24, 332–336.
Sugimoto, Y., Furutani, S., Itoh, A., Tanahashi, T., Nakajima, H., Oshiro, H., et al. (2008). Effects of extracts and neferine from the embryo of Nelumbo nucifera seeds on the central nervous system. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 15, 1117–1124.
Poornima, P., Quency, R. S., & Padma, V. V. (2013). Neferine induces reactive oxygen species mediated intrinsic pathway of apoptosis in HepG2 cells. Food Chemistry, 136, 659–667.
Cao, J. G., Tang, X. Q., & Shi, S. H. (2004). Multidrug resistance reversal in human gastric carcinoma cells by neferine. World Journal of Gastroenterology: WJG, 10, 3062–3064.
Li, G. R., Qian, J. Q., & Lu, F. H. (1990). Effects of neferine on heart electromechanical activity in anaesthetized cats. Zhongguo yao li xue bao = Acta Pharmacologica Sinica, 11, 158–161.
Yu, J., & Hu, W. S. (1997). [Effects of neferine on platelet aggregation in rabbits]. Yao xue xue bao = Acta pharmaceutica Sinica, 32, 1–4.
Feng, Y., Wu, J., Cong, R., Wang, C., Zong, Y., & Feng, Z. (1998). The effect of neferine on foam cell formation by anti-low density lipoprotein oxidation. Journal of Tongji Medical University = Tong ji yi ke da xue xue bao, 18, 134–136.
Wu, S., Sun, C., Cao, X., Zhou, H., Hong, Z., & Pan, Y. (2004). Preparative counter-current chromatography isolation of liensinine and its analogues from embryo of the seed of Nelumbo nucifera GAERTN. Using upright coil planet centrifuge with four multilayer coils connected in series. Journal of Chromatography A, 1041, 153–162.
Zhou, R., Xu, Q., Zheng, P., Yan, L., Zheng, J., & Dai, G. (2008). Cardioprotective effect of fluvastatin on isoproterenol-induced myocardial infarction in rat. European Journal of Pharmacology, 586, 244–250.
Okinaka, S., Kumagai, H., Ebashi, S., Sugita, H., Momoi, H., Toyokura, Y., et al. (1961). Serum creatine phosphokinase. Activity in progressive muscular dystrophy and neuromuscular diseases. Archives of Neurology, 4, 520–525.
King, J., (1965). The dehydrogenase of oxidoreductase–lactate dehydrogenase. Van, D. Nostrand Company, London.
Bergmeyer, H. U., Bernt, E., Mollering, H., & Fleiderer, G. (1974). l-Aspartate and l-asparagine in methods of enzymatic analysis. Journal of Biochemistry, 4, 1696–1700.
Parekh, A. C., & Jung, D. H. (1970). Cholesterol determination with ferric acetate-uranium acetate and sulfuric acid-ferrous sulfate reagents. Analytical Chemistry, 42, 1423–1427.
Rouser, G., Fleischer, S., & Yamamoto, A. (1970). Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids, 5, 494–496.
Rice, E. W. (1970). Standard method of clinical chemistry. New York: Academic Press.
Burstein, M., & Scholnick, H. R. (1973). Lipoprotein-polyanion-metal interactions. Advances in Lipid Research, 11, 67–108.
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
William, D. W. (1979). A more sensitive assay discriminating galactosamine and glucosamine in mixtures. Analytical Biochemistry, 94, 394–396.
Dische, Z., & Shettles, L. B. (1948). A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. Journal of Biological Chemistry, 175, 595–603.
Warren, L. (1959). The thiobarbituric acid assay of sialic acids. The Journal of biological chemistry, 234, 1971–1975.
Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95, 351–358.
Marklund, S., & Marklund, G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry/FEBS, 47, 469–474.
Takahara, S., Hamilton, H. B., Neel, J. V., Kobara, T. Y., Ogura, Y., & Nishimura, E. T. (1960). Hypocatalasemia: A new genetic carrier state. The Journal of Clinical Investigation, 39, 610–619.
Moron, M.S., Depierre, J.W. and Mannervik, B. (1979). Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochimica et Biophysica Acta (BBA)-General Subjects 582:67–78.
Tietze, F. (1969). Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Analytical Biochemistry, 27, 502–522.
Rotruck, J. T., Pope, A. L., Ganther, H. E., Swanson, A. B., Hafeman, D. G., & Hoekstra, W. G. (1973). Selenium: Biochemical role as a component of glutathione peroxidase. Science, 179, 588–590.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferases. Journal of Biological Chemistry, 249, 7130–7139.
Folch, J., Lees, M., & Stanley, G. H. S. (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226, 497–509.
Haenen, G. R. M. M., Veerman, M., & Bast, A. (1990). Reduction of β-adrenoceptor function by oxidative stress in the heart. Free Radical Biology and Medicine, 9, 279–288.
Upaganlawar, A., Gandhi, C., & Balaraman, R. (2009). Effect of green tea and vitamin E combination in isoproterenol induced myocardial infarction in rats. Plant Foods for Human Nutrition, 64, 75–80.
Judd, J. T., & Wexler, B. C. (1974). Myocardial glycoprotein changes with isoproterenol-induced necrosis and repair in the rat. American Journal of Physiology, 226, 597–602.
Patel, V., Upaganlawar, A., Zalawadia, R., & Balaraman, R. (2010). Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: A biochemical, electrocardiographic and histoarchitectural evaluation. European Journal of Pharmacology, 644, 160–168.
Sabeena Farvin, K. H., Anandan, R., Senthil Kumar, S. H., Shiny, K. S., Sankar, T. V., & Thankappan, T. K. (2004). Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacological Research, 50, 231–236.
Gürgün, C., Ildızlı, M., Yavuzgil, O., Sin, A., Apaydın, A., Çınar, C., et al. (2008). The effects of short term statin treatment on left ventricular function and inflammatory markers in patients with chronic heart failure. International Journal of Cardiology, 123, 102–107.
Prabhu, S., Jainu, M., Sabitha, K. E., & Devi, C. S. (2006). Cardioprotective effect of mangiferin on isoproterenol induced myocardial infarction in rats. Indian Journal of Experimental Biology, 44, 209–215.
Yang, Y., Shui, Q. L., Zeng, X. R., Liu, Z. F., Zhou, W., & Li, M. L. (2000). Effects of neferine on potassium channels in guinea pig ventricular cells and porcine coronary artery smooth muscle cells. Chinese Journal of Pharmacology and Toxicology, 14, 405–410.
Tang, X., & Cao, J. G. (2004). Review on the pharmacological research of neferine. Chinese Pharmacology Bulletin, 20, 8–10.
Singal, P. K., Kapur, N., Dhillon, K. S., Beamish, R. E., & Dhalla, N. S. (1982). Role of free radicals in catecholamine-induced cardiomyopathy. Canadian Journal of Physiology and Pharmacology, 60, 1390–1397.
Sevanian, A., & Hochstein, P. (1985). Mechanisms and consequences of lipid peroxidation in biological systems. Annual Review of Nutrition, 5, 365–390.
Hari Senthil Kumar, S., Anandan, R., Devaki, T., & Santhosh Kumar, M. (2001). Cardioprotective effects of Picrorhiza kurroa against isoproterenol-induced myocardial stress in rats. Fitoterapia, 72, 402–405.
Jayalakshmi, R., & Niranjali Devaraj, S. (2004). Cardioprotective effect of tincture of crataegus on isoproterenol-induced myocardial infarction in rats. The Journal of Pharmacy and Pharmacology, 56, 921–926.
Rai, S., Wahile, A., Mukherjee, K., Saha, B. P., & Mukherjee, P. K. (2006). Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. Journal of Ethnopharmacology, 104, 322–327.
Jayalakshmi, R., Thirupurasundari, C., & Devaraj, S. (2006). Pretreatment with alcoholic extract of shape Crataegus oxycantha (AEC) activates mitochondrial protection during isoproterenol-induced myocardial infarction in rats. Molecular and Cellular Biochemistry, 292, 59–67.
Ithayarasi, A. P., & Devi, C. S. (1997). Effect of alpha-tocopherol on lipid peroxidation in isoproterenol induced myocardial infarction in rats. Indian Journal of Physiology and Pharmacology, 41, 369–376.
Ji, L. L., Stratman, F. W., & Lardy, H. A. (1988). Antioxidant enzyme systems in rat liver and skeletal muscle: Influences of selenium deficiency, chronic training, and acute exercise. Archives of Biochemistry and Biophysics, 263, 150–160.
Priscilla, D. H., & Prince, P. S. M. (2009). Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chemico-Biological Interactions, 179, 118–124.
Zhao, L., Wang, X., Chang, Q., Xu, J., Huang, Y., Guo, Q., et al. (2010). Neferine, a bisbenzylisoquinoline alkaloid attenuates bleomycin-induced pulmonary fibrosis. European Journal of Pharmacology, 627, 304–312.
Mathew, S., Menon, P. V., & Kurup, P. A. (1981). Changes in myocardial & aortic lipids, lipolytic activity & fecal excretion of sterols & bile acids in isoproterenol-induced myocardial infarction in rats. Indian Journal of Biochemistry & Biophysics, 18, 131–133.
Sathish, V., Kumar Ebenezar, K., & Devaki, T. (2003). Synergistic effect of nicorandil and amlodipine on tissue defense system during experimental myocardial infarction in rats. Molecular and Cellular Biochemistry, 243, 133–138.
Jacksen, G. (1998). Metabolic agents for stable angina.
Karthikeyan, K., Sarala Bai, B. R., & Niranjali Devaraj, S. (2007). Efficacy of grape seed proanthocyanidins on serum and heart tissue lipids in rats subjected to isoproterenol-induced myocardial injury. Vascular Pharmacology, 47, 295–301.
Manjula, T. S., Geetha, A., Ramesh, T. G., & Devi, C. S. (1992). Reversal of changes of myocardial lipids by chronic administration of aspirin in isoproterenol-induced myocardial damage in rats. Indian Journal of Physiology and Pharmacology, 36, 47–50.
Yeagle, P. L. (1985). Cholesterol and the cell membrane. Biochimica et Biophysica Acta, 822, 267–287.
Zern, T. L., West, K. L., & Fernandez, M. L. (2003). Grape polyphenols decrease plasma triglycerides and cholesterol accumulation in the aorta of ovariectomized guinea pigs. The Journal of Nutrition, 133, 2268–2272.
Punithavathi, V. R., & Prince, P. S. M. (2009). Combined effects of quercetin and α-tocopherol on lipids and glycoprotein components in isoproterenol induced myocardial infarcted Wistar rats. Chemico-Biological Interactions, 181, 322–327.
Judd, J. T., & Wexler, B. C. (1970). Myocardial connective tissue metabolism in response to injury: II. Investigation of the mucopolysaccharides involved in isoproterenol-induced necrosis and repair in rat hearts. Circulation Research, 26, 101–109.
Acknowledgments
The authors express their sincere gratitude to Mr. Kumaresh, Sanjeevini Herbals, Salem, India, for providing lotus seeds for the isolation of neferine.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lalitha, G., Poornima, P., Archanah, A. et al. Protective Effect of Neferine Against Isoproterenol-Induced Cardiac Toxicity. Cardiovasc Toxicol 13, 168–179 (2013). https://doi.org/10.1007/s12012-012-9196-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-012-9196-5