Abstract
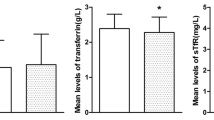
The objective of this study was to investigate the potential associations between serum iron levels, dietary iron intake, and iron supplementation, and the prevalence of metabolic syndrome (MetS) in adolescents A cross-sectional analysis was conducted, utilizing data from adolescents participating in the 2003–2018 cycle of the National Health and Nutrition Examination Survey (NHANES). Odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) pertaining to serum iron, dietary iron, and iron supplementation were derived through multivariate logistic regression models. Additionally, a restricted cubic spline (RCS) regression model was applied to explore the nonlinear relationship between dietary iron and serum iron concerning MetS. The study encompassed 4858 American adolescents aged 12 to 19, among whom 413 (8.5%) manifested MetS. The study cohort exhibited an average age of 15.52 years, comprising 2551 males (52.51%) and 2307 females (47.49%). Relative to individuals in the lowest serum iron quartile, those in the highest quartile for serum iron (OR = 0.33, 95% CI 0.21–0.50), the highest quartile for dietary iron (OR = 0.53, 95% CI 0.32–0.89), and those utilizing iron supplements (OR = 0.61, 95% CI 0.37–0.99) evinced a diminished prevalence of MetS, even post adjustment for potential confounding variables. A non-linear relationship was discerned between serum iron and MetS, exhibiting a statistically significant negative correlation when serum iron concentrations exceeded the inflection point (serum iron = 8.66 µmol/L, P for nonlinear < 0.001). This investigation reveals that higher levels of serum iron, increased dietary iron intake, and the use of iron supplements are linked to a lower prevalence of MetS in US adolescents. These findings suggest that dietary modifications could play a role in promoting the health of adolescents.


Similar content being viewed by others
Data Availability
NHANES data described in this manuscript are available at https://wwwn.cdc.gov/nchs/nhanes/.
References
DeBoer MD (2019) Assessing and managing the metabolic syndrome in children and adolescents. Nutrients 11(8):1788
Yang L, Cao C, Kantor ED, Nguyen LH, Zheng X, Park Y, Giovannucci EL, Matthews CE, Colditz GA, Cao Y (2019) Trends in sedentary behavior among the US population, 2001–2016. JAMA 321(16):1587–1597
Liao J, Cao C, Hur J, Cohen J, Chen W, Zong X, Colditz G, Yang L, Stamatakis E, Cao Y (2021) Association of sedentary patterns with body fat distribution among US children and adolescents: a population-based study. Int J Obes 45(9):2048–2057
Gaston SA, Tulve NS (2019) Urinary phthalate metabolites and metabolic syndrome in U.S. adolescents: cross-sectional results from the National Health and Nutrition Examination Survey (2003–2014) data. Int J Hyg Environ Health 222(2):195–204
Pogodina A, Rychkova L, Kravtzova O, Klimkina J, Kosovtzeva A (2017) Cardiometabolic risk factors and health-related quality of life in adolescents with obesity. Child Obes 13(6):499–506
Bovolini A, Garcia J, Andrade MA, Duarte JA (2021) Metabolic syndrome pathophysiology and predisposing factors. Int J Sports Med 42(3):199–214
Mahajan N, Kshatriya GK (2020) Prevalence of metabolic syndrome and associated risk factors among tribal adolescents of Gujarat. Diabetes Metab Syndr 14(5):995–999
Siwarom S, Aekplakorn W, Pirojsakul K, Paksi W, Kessomboon P, Neelapaichit N, Chariyalertsak S, Assanangkornchai S, Taneepanichskul S (2021) Metabolic syndrome in Thai adolescents and associated factors: the Thai National Health Examination Survey V (NHES V). BMC Public Health 21(1):678
Zhu Z, He Y, Wu F, Zhao L, Wu C, Lu Y, Zang J, Wang Z, Sun J, Huang J et al (2020) The associations of dietary iron, zinc and magnesium with metabolic syndrome in China’s mega cities. Nutrients 12(3)
Śliwińska A, Luty J, Aleksandrowicz-Wrona E, Małgorzewicz S (2018) Iron status and dietary iron intake in vegetarians. Adv Clin Exp Med 27(10):1383–1389
Timoteo VJ, Chiang KM, Pan WH (2022) Positive or u-shaped association of elevated hemoglobin concentration levels with metabolic syndrome and metabolic components: findings from Taiwan Biobank and UK Biobank. Nutrients 14(19):4007
Hashimoto Y, Tanaka M, Kimura T, Kitagawa N, Hamaguchi M, Asano M, Yamazaki M, Oda Y, Toda H, Nakamura N, Fukui M (2015) Hemoglobin concentration and incident metabolic syndrome: a population-based large-scale cohort study. Endocrine 50(2):390–396
Chen TC, Clark J, Riddles MK, Mohadjer LK, Fakhouri THI (2020) National Health and Nutrition Examination Survey, 2015–2018: sample design and estimation procedures. Vital Health Stat 2(184):1–35.
Alberti KG, Zimmet P, Shaw J (2006) Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med 23(5):469–480
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 285(19):2486–2497
Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T (2009) Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol 4(12):1901–1906
Zhu X, Cheang I, Tang Y, Shi M, Zhu Q, Gao R, Liao S, Yao W, Zhou Y, Zhang H, Li X (2023) Associations of serum carotenoids with risk of all-cause and cardiovascular mortality in hypertensive adults. J Am Heart Assoc 12(4):e027568
Wahl R (1999) Nutrition in the adolescent. Pediatr Ann 28(2):107–111
Corley RP, Beltz AM, Wadsworth SJ, Berenbaum SA (2015) Genetic influences on pubertal development and links to behavior problems. Behav Genet 45(3):294–312
Lassi ZS, Mansoor T, Salam RA, Bhutta SZ, Das JK, Bhutta ZA (2017) Review of nutrition guidelines relevant for adolescents in low- and middle-income countries. Ann N Y Acad Sci 1393(1):51–60
Suárez-Ortegón MF, Blanco E, McLachlan S, Fernandez-Real JM, Burrows R, Wild SH, Lozoff B, Gahagan S (2019) Ferritin levels throughout childhood and metabolic syndrome in adolescent stage. Nutr Metab Cardiovasc Dis 29(3):268–278
Zhang C, Rawal S (2017) Dietary iron intake, iron status, and gestational diabetes. Am J Clin Nutr 106(Suppl 6):1672s–1680s
Liu J, Li Q, Yang Y, Ma L (2020) Iron metabolism and type 2 diabetes mellitus: a meta-analysis and systematic review. J Diabetes Investig 11(4):946–955
Fang C, Wu W, Gu X, Dai S, Zhou Q, Deng H, Shen F, Chen J (2019) Association of serum copper, zinc and selenium levels with risk of metabolic syndrome: a nested case-control study of middle-aged and older Chinese adults. J Trace Elem Med Biol 52:209–215
González-Domínguez Á, Visiedo-García FM, Domínguez-Riscart J, González-Domínguez R, Mateos RM, Lechuga-Sancho AM (2020) Iron metabolism in obesity and metabolic syndrome. Int J Mol Sci 21(15):5529
Wang M (2016) Iron deficiency and other types of anemia in infants and children. Am Fam Physician 93(4):270–278
Ogunsile FJ, Bediako SM, Nelson J, Cichowitz C, Yu T, Patrick Carroll C, Stewart K, Naik R, Haywood C Jr, Lanzkron S (2019) Metabolic syndrome among adults living with sickle cell disease. Blood Cells Mol Dis 74:25–29
Zhu Y, He B, Xiao Y, Chen Y (2019) Iron metabolism and its association with dyslipidemia risk in children and adolescents: a cross-sectional study. Lipids Health Dis 18(1):50
Zhu Y, Chen G, Bo Y, Liu Y (2019) Markers of iron status, blood pressure and incident hypertension among Chinese adults. Nutr Metab Cardiovasc Dis 29(8):830–836
Urrechaga E (2018) Influence of iron deficiency on Hb A1c levels in type 2 diabetic patients. Diabetes Metab Syndr 12(6):1051–1055
Chaudhari AS, Raghuvanshi R, Kumar GN (2017) Genetically engineered Escherichia coli Nissle 1917 synbiotic counters fructose-induced metabolic syndrome and iron deficiency. Appl Microbiol Biotechnol 101(11):4713–4723
Harrison AV, Lorenzo FR, McClain DA (2023) Iron and the pathophysiology of diabetes. Annu Rev Physiol 85:339–362
Galaris D, Barbouti A, Pantopoulos K (2019) Iron homeostasis and oxidative stress: an intimate relationship. Biochim Biophys Acta, Mol Cell Res 1866(12):118535
Imam MU, Zhang S, Ma J, Wang H, Wang F (2017) Antioxidants mediate both iron homeostasis and oxidative stress. Nutrients 9(7):671
Intracellular iron transport and storage (2008) from molecular mechanisms to health implications. Antioxid Redox Signal 10(6):997–1030
Jaccard E, Seyssel K, Gouveia A, Vergely C, Baratali L, Gubelmann C, Froissart M, Favrat B, Marques-Vidal P, Tappy L, Waeber G (2022) Effect of acute iron infusion on insulin secretion: a randomized, double-blind, placebo-controlled trial. EClinicalMedicine 48:101434
Datz C, Felder TK, Niederseer D, Aigner E (2013) Iron homeostasis in the metabolic syndrome. Eur J Clin Invest 43(2):215–224
Sachinidis A, Doumas M, Imprialos K, Stavropoulos K, Katsimardou A, Athyros VG (2020) Dysmetabolic iron overload in metabolic syndrome. Curr Pharm Des 26(10):1019–1024
Acknowledgements
We appreciate the people who contributed to the NHANES data we studied. Moreover, thanks to Zhang Jing (Shanghai Tongren Hospital) for his work on the NHANES database.
His outstanding work, nhanesR package and webpage, makes it easier for us to explore NHANES database.
Author information
Authors and Affiliations
Contributions
Meng Wang: conceptualization, project administration, methodology, software, formal analysis, writing—original draft, visualization. Zhiyuan Chen: conceptualization, methodology, writing—review and editing, conceptualization, supervision. Yuanfeng Zhang: writing—review and editing, supervision.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All participants provided written informed consent, and study procedures were approved by the National Center for Health Statistics Research Ethics Review Board (Protocol Number: Protocol #98–12, Protocol #2005–06, and Protocol #2011–17).
Consent for Publication
The manuscript is approved by all authors for publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, M., Chen, Z. & Zhang, Y. Serum Iron Levels, Dietary Iron Intake, and Supplement Use in Relation to Metabolic Syndrome in Adolescents: A Cross-Sectional Study. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04152-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04152-1