Abstract
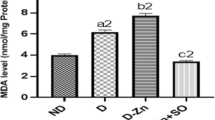
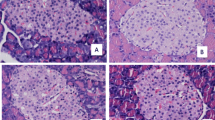
Boron (B) is an element that has recently been wondered and researched in many fields, especially due to its effects on energy metabolism. The aim of this study is to evaluate the effect of boric acid (BA) on newly discovered energy metabolism peptides that have not been studied before. In this study, the effects of 15 mg/kg of BA were evaluated in 24 Wistar rats. Groups were named as control group, 15 mg/kg BA group, streptozotocin (STZ)-induced experimental diabetic group, and STZ-induced experimental diabetic + 15 mg/kg BA administered group (STZ+15 mg/kg BA). Serum asprosin, nesfatin-1, preptin, insulin, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), aspartate transaminase (AST), alanine transaminase (ALT), and glucose analyses were performed. In this study, the increase in glucose, TG, TC, LDL-C levels, and AST, ALT activities in STZ-induced groups were reduced with BA administration. While HDL-C level significantly decreased in the STZ group, the level approached the control group values after BA administration (p<0.001). As for peptides, although there was a statistically significant increase after 15 mg/kg BA administration, these levels did not approach the control group values (p<0.001). According to the findings, STZ-induced diabetes mellitus and the biochemical processes that develop accordingly change correlatively. This study showed that BA is effective in energy metabolism.




Similar content being viewed by others
Data Availability
No datasets were generated during the current study.
Change history
10 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12011-023-03944-1
References
Tomic D, Shaw JE, Magliano DJ (2022) The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol 18:525–539. https://doi.org/10.1038/s41574-022-00690-7
Farrin N, Rezazadeh L, Pourmoradian S et al (2022) Boron compound administration; A novel agent in weight management: A systematic review and meta- analysis of animal studies. J Trace Elem Med Biol 72:126969. https://doi.org/10.1016/j.jtemb.2022.126969
WHO (1998) Boron. International Programme on Chemical Safety. Environmental Health Criteria 204. Ohio, p 1–201
Ri CC, CRDRV M et al (2023) Boron-Containing Compounds for Prevention, Diagnosis, and Treatment of Human Metabolic Disorders. Biol Trace Elem Res 201:2222–2239. https://doi.org/10.1007/s12011-022-03346-9
Kan F, Kucukkurt I (2023) The Effects of Boron on Some Biochemical Parameters: A Review. J Trace Elem Med Biol 79:127249. https://doi.org/10.1016/j.jtemb.2023.127249
Nielsen FH (2014) Update on human health effects of boron. J Trace Elem Med Biol 28:383–387. https://doi.org/10.1016/j.jtemb.2014.06.023
Scorei R (2006) Boron, essential micronutrient for animal nutrition. Analele IBNA 22:75–85
Kabu M, Birdane FM, Civelek T, Uyarlar C (2013) Affects of boron administration on serum Ca, Mg and P ofperipartum Cows. Arch Anim Breed 56:733–741. https://doi.org/10.7482/0003-9438-56-073
Sheng MHC, Taper LJ, Veit H et al (2001) Dietary boron supplementation enhances the effects of estrogen on bone mineral balance in ovariectomized rats. Biol Trace Elem Res 81:29–45. https://doi.org/10.1385/BTER:81:1:29
Ince S, Keles H, Erdogan M et al (2012) Protective effect of boric acid against carbon tetrachloride-induced hepatotoxicity in mice. Drug Chem Toxicol 35:285–292. https://doi.org/10.3109/01480545.2011.607825
Cakir S, Eren M, Senturk M, Sarica ZS (2018) The Effect of Boron on Some Biochemical Parameters in Experimental Diabetic Rats. Biol Trace Elem Res 184:165–172. https://doi.org/10.1007/s12011-017-1182-0
Basbug M, Yildar M, Yaman I et al (2015) Effects of boric acid in an experimental rat model of hepatic ischemia-reperfusion injury. Acta Medica Mediterr 31:1067–1073
Hunt CD (1998) Regulation of Enzymatic ActivityOne Possible Role of Dietary Boron in Higher Animals and Humans. Pyruvate Carboxylase. Biol Trace Elem Res 66:141–177. https://doi.org/10.1201/9781351076142-5
Romere C, Duerrschmid C, Bournat J et al (2016) Asprosin, a Fasting-Induced Glucogenic Protein Hormone. Cell 165:566–579. https://doi.org/10.1016/J.CELL.2016.02.063
Duerrschmid C, He Y, Wang C et al (2017) Asprosin is a centrally acting orexigenic hormone. Nat med. https://doi.org/10.1038/nm.4432
Ceylan Hİ, Saygın Ö (2021) An investigation of the relationship between new fasting hormone asprosin, obesity and acute-chronic exercise: current systematic review. Arch Physiol Biochem 127(4):373–384. https://doi.org/10.1080/13813455.2020.1767652
Oh-I S, Shimizu H, Satoh T et al (2006) Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443:709–712. https://doi.org/10.1038/nature05162
Sun H, Zhao H, Yan Z et al (2021) Protective role and molecular mechanism of action of Nesfatin-1 against high glucose-induced inflammation, oxidative stress and apoptosis in retinal epithelial cells. Exp Ther Med 22:1–8. https://doi.org/10.3892/etm.2021.10265
Buchanan CM, Phillips ARJ, Cooper GJS (2001) Preptin derived from proinsulin-like growth factor II (proIGF-II) is secreted from pancreatic islet β-cells and enhances insulin secretion. Biochem J 360:431–439. https://doi.org/10.1042/0264-6021:3600431
Aydin S (2014) Three new players in energy regulation: Preptin, adropin and irisin. Peptides 56:94–110. https://doi.org/10.1016/j.peptides.2014.03.021
Coban FK, Liman R, Cigerci IH et al (2015) The antioxidant effect of boron on oxidative stress and DNA damage in diabetic rats. Fresenius Environ Bull 24:4059–4066
Ar’Rajab A, Ahrén B (1993) Long-term diabetogenic effect of streptozotocin in rats. Pancreas 8:50–57. https://doi.org/10.1097/00006676-199301000-00011
Smallwood CL, Lipscomb J, Swartout J, Teuschler L (2010) Toxicological Review of Boron and Compounds. US Environ Prot Agency 39:759–786
Devirian TA, Volpe SL (2003) The Physiological Effects of Dietary Boron. Crit Rev Food Sci Nutr 43:219–231. https://doi.org/10.1080/10408690390826491
Nielsen FH (2014) Should bioactive trace elements not recognized as essential, but with beneficial health effects, have intake recommendations. J Trace Elem Med Biol 28:406–408. https://doi.org/10.1016/j.jtemb.2014.06.019
Hunt CD (1989) Dietary boron modified the effects of magnesium and molybdenum on mineral metabolism in the cholecalciferol-deficient chick. Biol Trace Elem Res 22:201–220. https://doi.org/10.1007/BF02916650
Kan F, Kucukkurt I (2022) Investigation of the effect of boron on thyroid functions and biochemical parameters in hypothyroid induced-rats. J Biochem Mol Toxicol. https://doi.org/10.1002/jbt.23186
Kucukkurt I, Akbel E, Karabag F, Ince S (2015) The effects of dietary boron compounds in supplemented diet on hormonal activity and some biochemical parameters in rats. Toxicol Ind Health 31:255–260. https://doi.org/10.1177/0748233712469648
Demirdogen RE (2020) Relationship Among Blood Boron Level, Diabetes Mellitus, Lipid Metabolism, Bone Metabolism and Obesity: Can Boron Be An Efficient Indicator for Metabolic Dissesases? Heal Sci J 14:1–11. https://doi.org/10.36648/1791-809x.14.1.689
Hunt CD, Herbel JL (1991) Effects of dietary boron on calcium and mineral metabolism in the streptozotocin-injected, vitamin D3-deprived rat. Magnes Trace Elem 92(10):387–408
López-Cabrera Y, Castillo-García EL, Altamirano-Espino JA et al (2018) Profile of three boron-containing compounds on the body weight, metabolism and inflammatory markers of diabetic rats. J Trace Elem Med Biol 50:424–429. https://doi.org/10.1016/j.jtemb.2018.08.009
Aysal H, Atasoy N, Kömüroğlu AU (2023) Protective Effect of Calcium Fructoborate Against Carbon Tetrachloride–Induced Toxicity in Rats. Biol Trace Elem Res 201:800–809. https://doi.org/10.1007/s12011-022-03202-w
Kikuchi H, Nakamura Y, Inoue C et al (2021) Hydrogen Peroxide-Triggered Conversion of Boronic Acid-Appended Insulin into Insulin and Its Application as a Glucose-Responsive Insulin Formulation. Mol Pharm 18:4224–4230. https://doi.org/10.1021/acs.molpharmaceut.1c00760
Aydın S, Demirci S, Doğan A et al (2019) Boron containing compounds promote the survival and the maintenance of pancreatic β-cells. Mol Biol Rep 46:5465–5478. https://doi.org/10.1007/s11033-019-05002-3
Ozel AB, Dagsuyu E, Aydın PK et al (2022) Brain Boron Level, DNA Content, and Myeloperoxidase Activity of Metformin-Treated Rats in Diabetes and Prostate Cancer Model. Biol Trace Elem Res 200:1164–1170. https://doi.org/10.1007/s12011-021-02708-z
Bekaert S, Derradji H, Baatout S (2004) Telomere biology in mammalian germ cells and during development. Dev Biol 274:15–30. https://doi.org/10.1016/j.ydbio.2004.06.023
Gonzalez R, Reingold BK, Gao X et al (2011) Nesfatin-1 exerts a direct, glucose-dependent insulinotropic action on mouse islet β- and MIN6 cells. J Endocrinol 208. https://doi.org/10.1530/JOE-10-0492
Nakata M, Manaka K, Yamamoto S et al (2011) Nesfatin-1 enhances glucose-induced insulin secretion by promoting Ca2+ influx through L-type channels in mouse islet β-cells. Endocr J 58:305–313. https://doi.org/10.1507/endocrj.K11E-056
Foo KS, Brauner H, Östenson CG, Broberger C (2010) Nucleobindin-2/nesfatin in the endocrine pancreas: Distribution and relationship to glycaemic state. J Endocrinol 204:255–263. https://doi.org/10.1677/JOE-09-0254
Duan H, Ma B, Ma X et al (2016) Anti-diabetic activity of recombinant irisin in STZ-induced insulin-deficient diabetic mice. Int J Biol Macromol 84:457–463. https://doi.org/10.1016/j.ijbiomac.2015.12.049
Cornish J, Callon KE, Bava U et al (2007) Preptin, another peptide product of the pancreatic β-cell, is osteogenic in vitro and in vivo. Am J Physiol - Endocrinol Metab 292. https://doi.org/10.1152/ajpendo.00642.2005
Moustafa A, Arisha AH (2020) Swim therapy-induced tissue specific metabolic responses in male rats. Life Sci 262:118516. https://doi.org/10.1016/J.LFS.2020.118516
Kocaman N, Kuloğlu T (2020) Expression of asprosin in rat hepatic, renal, heart, gastric, testicular and brain tissues and its changes in a streptozotocin-induced diabetes mellitus model. Tissue Cell 66:101397. https://doi.org/10.1016/J.TICE.2020.101397
Wang Y, Qu H, Xiong X et al (2018) Plasma Asprosin Concentrations Are Increased in Individuals with Glucose Dysregulation and Correlated with Insulin Resistance and First-Phase Insulin Secretion. Mediators Inflamm. https://doi.org/10.1155/2018/9471583
Sünnetçi Silistre E, Hatipoğl HU (2020) Increased serum circulating asprosin levels in children with obesity. Pediatr Int 62:467–476. https://doi.org/10.1111/ped.14176
Ko JR, Seo DY, Kim TN et al (2019) Aerobic exercise training decreases hepatic asprosin in diabetic rats. J Clin Med 8. https://doi.org/10.3390/jcm8050666
Farrag M, Ait Eldjoudi D, González-Rodríguez M et al (2023) Asprosin in health and disease, a new glucose sensor with central and peripheral metabolic effects. Front Endocrinol (Lausanne) 13:1–17. https://doi.org/10.3389/fendo.2022.1101091
Author information
Authors and Affiliations
Contributions
Selcen CAKIR wrote the manuscript and conducted experiments, analyzed the data, and revised the manuscript. The author approved the final draft.
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by Çanakkale Onsekiz Mart University Animal Experiments Local Ethics Committee (ÇOMÜ HADYEK 30.06.2020; 2020/06-05).
Competing Interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Further studies are required to better monitor the effect of BA on energy metabolism.
The original online version of this article was revised: the tables and figures have been updated
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
ÇAKIR, S. Effect of Boric Acid on Metabolic Peptides and Some Biochemical Parameters in Experimental Diabetic Rats. Biol Trace Elem Res 202, 1001–1008 (2024). https://doi.org/10.1007/s12011-023-03910-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03910-x