Abstract
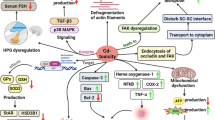
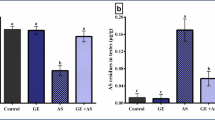
Long-term exposure to arsenic can lead to testicular damage and lower sperm quality in males, which is mediated by increased arsenic-induced oxidative stress and other damage mechanisms. D-Limonene, which is rich in oranges, lemons, oranges, grapes and other natural fruits, can relieve doxorubicin (DOX)-induced kidney injury and CCL4-induced cardiac toxicity by inhibiting oxidative stress and inflammatory response. The antioxidant and anti-inflammatory properties of D-limonene motivate us to further explore whether it can reduce arsenic-induced testicular injury. To verify this scientific hypothesis, testicular pathology, testicular oxidative stress levels and sperm motility were determined after intervention with D-limonene in rats chronically exposed to arsenic. As expected, long-term arsenic exposure caused testicular tissue structure disturbances, increased levels of oxidative stress, and decreased sperm activation, all of which were significantly inhibited due to treatment with D-limonene. In conclusion, our data reveal a previously unproven beneficial effect of D-limonene, namely that D-limonene can inhibit arsenic-induced testicular injury, and also provide theoretical and experimental basis for the application of D-limonene in the treatment of arsenic-induced testicular injury.




Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Rahaman MS, Rahman MM, Mise N et al (2021) Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ Pollut (Barking, Essex : 1987) 289:117940. https://doi.org/10.1016/j.envpol.2021.117940
Nurchi VM, Djordjevic AB, Crisponi G et al (2020) Arsenic toxicity: molecular targets and therapeutic agents. Biomolecules 10. https://doi.org/10.3390/biom10020235
Ratnaike RN (2003) Acute and chronic arsenic toxicity. Postgrad Med J 79:391–396. https://doi.org/10.1136/pmj.79.933.391
Sinha D, Prasad P (2020) Health effects inflicted by chronic low-level arsenic contamination in groundwater: a global public health challenge. J Appl Toxicol : JAT 40:87–131. https://doi.org/10.1002/jat.3823
Huang Q, Luo L, Alamdar A et al (2016) Integrated proteomics and metabolomics analysis of rat testis: mechanism of arsenic-induced male reproductive toxicity. Scientific reports 6:32518. https://doi.org/10.1038/srep32518
Das J, Ghosh J, Manna P et al (2009) Taurine protects rat testes against NaAsO(2)-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicol Lett 187:201–210. https://doi.org/10.1016/j.toxlet.2009.03.001
Pant N, Murthy RC, Srivastava SP (2004) Male reproductive toxicity of sodium arsenite in mice. Hum Exp Toxicol 23:399–403. https://doi.org/10.1191/0960327104ht467oa
Reddy PS, Rani GP, Sainath SB et al (2011) Protective effects of N-acetylcysteine against arsenic-induced oxidative stress and reprotoxicity in male mice. J Trace Elem Med Biol 25:247–253. https://doi.org/10.1016/j.jtemb.2011.08.145
Zeng Q, Yi H, Huang L et al (2019) Long-term arsenite exposure induces testicular toxicity by redox imbalance, G2/M cell arrest and apoptosis in mice. Toxicology 411:122–132. https://doi.org/10.1016/j.tox.2018.09.010
Nurchi VM, Cappai R, Chand K et al (2019) New strong extrafunctionalizable tris(3,4-HP) and bis(3,4-HP) metal sequestering agents: synthesis, solution and in vivo metal chelation. Dalton Trans (Cambridge, England : 2003) 48:16167–16183. https://doi.org/10.1039/c9dt02905b
Flora SJ, Bhadauria S, Kannan GM et al (2007) Arsenic induced oxidative stress and the role of antioxidant supplementation during chelation: a review. J Environ Biol 28:333–347
Rao CV, Pal S, Mohammed A et al (2017) Biological effects and epidemiological consequences of arsenic exposure, and reagents that can ameliorate arsenic damage in vivo. Oncotarget 8:57605–57621. https://doi.org/10.18632/oncotarget.17745
Bjorklund G, Mutter J, Aaseth J (2017) Metal chelators and neurotoxicity: lead, mercury, and arsenic. Arch Toxicol 91:3787–3797. https://doi.org/10.1007/s00204-017-2100-0
Yadav A, Flora SJ (2016) Nano drug delivery systems: a new paradigm for treating metal toxicity. Expert Opin Drug Deliv 13:831–841. https://doi.org/10.1517/17425247.2016.1160890
Hu Y, Li J, Lou B et al (2020) The role of reactive oxygen species in arsenic toxicity. Biomolecules 10. https://doi.org/10.3390/biom10020240
Yaribeygi H, Sathyapalan T, Atkin SL et al (2020) Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid Med Cell Longev 2020:8609213. https://doi.org/10.1155/2020/8609213
Khosravi M, Poursaleh A, Ghasempour G et al (2019) The effects of oxidative stress on the development of atherosclerosis. Biol Chem 400:711–732. https://doi.org/10.1515/hsz-2018-0397
Qamar AY, Naveed MI, Raza S et al (2023) Role of antioxidants in fertility preservation of sperm - a narrative review. Anim Biosci 36:385–403. https://doi.org/10.5713/ab.22.0325
Scarlata E, O'Flaherty C (2020) Antioxidant enzymes and male fertility: lessons from knockout models. Antioxid Redox Signal 32:569–580. https://doi.org/10.1089/ars.2019.7985
Dutta S, Majzoub A, Agarwal A (2019) Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J Urol 17:87–97. https://doi.org/10.1080/2090598x.2019.1599624
Chen SJ, Allam JP, Duan YG et al (2013) Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet 288:191–199. https://doi.org/10.1007/s00404-013-2801-4
Agarwal A, Virk G, Ong C et al (2014) Effect of oxidative stress on male reproduction. World J Men’s Health 32:1–17. https://doi.org/10.5534/wjmh.2014.32.1.1
Bisht S, Faiq M, Tolahunase M et al (2017) Oxidative stress and male infertility. Nat Rev Urol 14:470–485. https://doi.org/10.1038/nrurol.2017.69
Tazari M, Baghshani H, Moosavi Z (2018) Effect of betaine versus arsenite-induced alterations of testicular oxidative stress and circulating androgenic indices in rats. Andrologia 50:e13091. https://doi.org/10.1111/and.13091
Ince S, Avdatek F, Demirel HH et al (2016) Ameliorative effect of polydatin on oxidative stress-mediated testicular damage by chronic arsenic exposure in rats. Andrologia 48:518–524. https://doi.org/10.1111/and.12472
Dehdashti Moghadam M, Baghshani H, Ghodrati Azadi H et al (2021) Ameliorative effects of caffeic acid against arsenic-induced testicular injury in mice. Biol Trace Elem Res 199:3772–3780. https://doi.org/10.1007/s12011-020-02518-9
González-Mas MC, Rambla JL, López-Gresa MP et al (2019) Volatile compounds in citrus essential oils: a comprehensive review. Front Plant Sci 10:12. https://doi.org/10.3389/fpls.2019.00012
Sharifi-Rad J, Sureda A, Tenore GC et al (2017) Biological activities of essential oils: from plant chemoecology to traditional healing systems. Molecules (Basel, Switzerland) 22. https://doi.org/10.3390/molecules22010070
Anandakumar P, Kamaraj S, Vanitha MK (2021) D-limonene: a multifunctional compound with potent therapeutic effects. J Food Biochem 45:e13566. https://doi.org/10.1111/jfbc.13566
Rehman MU, Tahir M, Khan AQ et al (2014) D-limonene suppresses doxorubicin-induced oxidative stress and inflammation via repression of COX-2, iNOS, and NFκB in kidneys of Wistar rats. Exp Biol Med (Maywood, NJ) 239:465–476. https://doi.org/10.1177/1535370213520112
Cellular Longevity OMA (2022) Retracted: protective effect of D-limonene against oxidative stress-induced cell damage in human lens epithelial cells via the p38 pathway. Oxid Med Cell Longev 2022:9862315. https://doi.org/10.1155/2022/9862315
AlSaffar RM, Rashid S, Ahmad SB et al (2022) D-limonene (5 (one-methyl-four-[1-methylethenyl]) cyclohexane) diminishes CCl(4)-induced cardiac toxicity by alleviating oxidative stress, inflammatory and cardiac markers. Redox Rep : Commun Free Radic Res 27:92–99. https://doi.org/10.1080/13510002.2022.2062947
Concessao PL, Bairy KL, Raghavendra AP (2023) Ameliorating effect of Mucuna pruriens seed extract on sodium arsenite-induced testicular toxicity and hepato-renal histopathology in rats. Vet World 16:82–93. https://doi.org/10.14202/vetworld.2023.82-93
Yan X, Chen X, Tian X et al (2021) Co-exposure to inorganic arsenic and fluoride prominently disrupts gut microbiota equilibrium and induces adverse cardiovascular effects in offspring rats. Sci Total Environ 767:144924. https://doi.org/10.1016/j.scitotenv.2020.144924
Na L, Xiumei Z et al (2020) Research into the intervention effect of folic acid on arsenic-induced heart abnormalities in fetal rats during the periconception period. BMC Cardiovasc Disord 20:139. https://doi.org/10.1186/s12872-020-01418-z
Huang Q, Xi G, Alamdar A et al (2017) Comparative proteomic analysis reveals heart toxicity induced by chronic arsenic exposure in rats. Environ Pollut (Barking, Essex : 1987) 229:210–218. https://doi.org/10.1016/j.envpol.2017.05.077
Mehta M, Hundal SS (2016) Effect of sodium arsenite on reproductive organs of female Wistar rats. Arch Environ Occup Health 71:16–25. https://doi.org/10.1080/19338244.2014.927346
Ferreira M, Matos RC, Oliveira H et al (2012) Impairment of mice spermatogenesis by sodium arsenite. Hum Exp Toxicol 31:290–302. https://doi.org/10.1177/0960327111405862
Smith LB, Walker WH (2014) The regulation of spermatogenesis by androgens. Semin Cell Dev Biol 30:2–13. https://doi.org/10.1016/j.semcdb.2014.02.012
Lin Y, Mahan K, Lathrop WF et al (1994) A hyaluronidase activity of the sperm plasma membrane protein PH-20 enables sperm to penetrate the cumulus cell layer surrounding the egg. J Cell Biol 125:1157–1163. https://doi.org/10.1083/jcb.125.5.1157
Hirayama T, Hasegawa T, Hiroi M (1989) The measurement of hyaluronidase activity in human spermatozoa by substrate slide assay and its clinical application. Fertil Steril 51:330–334. https://doi.org/10.1016/s0015-0282(16)60499-5
Zubair M, Ahmad M, Qureshi ZI (2017) Review on arsenic-induced toxicity in male reproductive system and its amelioration. Andrologia 49. https://doi.org/10.1111/and.12791
Jomova K, Jenisova Z, Feszterova M et al (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol : JAT 31:95–107. https://doi.org/10.1002/jat.1649
Chang SI, Jin B, Youn P et al (2007) Arsenic-induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicol Appl Pharmacol 218:196–203. https://doi.org/10.1016/j.taap.2006.11.009
White CC, Viernes H, Krejsa CM et al (2003) Fluorescence-based microtiter plate assay for glutamate-cysteine ligase activity. Anal Biochem 318:175–180. https://doi.org/10.1016/s0003-2697(03)00143-x
Zhao P, Guo Y, Zhang W et al (2017) Neurotoxicity induced by arsenic in gallus gallus: regulation of oxidative stress and heat shock protein response. Chemosphere 166:238–245. https://doi.org/10.1016/j.chemosphere.2016.09.060
Wirth JJ, Mijal RS (2010) Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med 56:147–167. https://doi.org/10.3109/19396360903582216
Fouad AA, Al-Sultan AI, Yacoubi MT (2011) Coenzyme Q10 counteracts testicular injury induced by sodium arsenite in rats. Eur J Pharmacol 655:91–98. https://doi.org/10.1016/j.ejphar.2010.12.045
Jana K, Jana S, Samanta PK (2006) Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible an estrogenic mode of action. Reprod Biol Endocrinol : RB & E 4:9. https://doi.org/10.1186/1477-7827-4-9
Kumar D, Panda SK, Jena GR et al (2023) Alternations of fertility parameters by graded dose of inorganic arsenic in adult male white Pekin ducks. Biol Trace Elem Res. https://doi.org/10.1007/s12011-023-03580-9
Jahan S, Iftikhar N, Ullah H et al (2015) Alleviative effect of quercetin on rat testis against arsenic: a histological and biochemical study. Syst Biol Reprod Med 61:89–95. https://doi.org/10.3109/19396368.2014.998350
El-Khadragy MF, Al-Megrin WA, Alomar S et al (2021) Chlorogenic acid abates male reproductive dysfunction in arsenic-exposed mice via attenuation of testicular oxido-inflammatory stress and apoptotic responses. Chem Biol Interact 333:109333. https://doi.org/10.1016/j.cbi.2020.109333
Kaspar JW, Niture SK, Jaiswal AK (2009) Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med 47:1304–1309. https://doi.org/10.1016/j.freeradbiomed.2009.07.035
Wajda A, Łapczuk J, Grabowska M et al (2016) Nuclear factor E2-related factor-2 (Nrf2) expression and regulation in male reproductive tract. Pharmacol Rep : PR 68:101–108. https://doi.org/10.1016/j.pharep.2015.07.005
Shaha C, Tripathi R, Mishra DP (2010) Male germ cell apoptosis: regulation and biology. Phil Trans R Soc Lond Series B, Biol Sci 365:1501–1515. https://doi.org/10.1098/rstb.2009.0124
Rojas-García PP, Recabarren MP, Sarabia L et al (2010) Prenatal testosterone excess alters Sertoli and germ cell number and testicular FSH receptor expression in rams. Am J Physiol Endocrinol Metab 299:E998–e1005. https://doi.org/10.1152/ajpendo.00032.2010
Zhao R, Yang B, Wang L et al (2013) Curcumin protects human keratinocytes against inorganic arsenite-induced acute cytotoxicity through an NRF2-dependent mechanism. Oxid Med Cell Longev 2013:412576. https://doi.org/10.1155/2013/412576
Li NC, Wei XX, Hu YL et al (2018) Aerobic exercise blocks interleukin-6 levels and germ cell apoptosis in obese rats. Andrologia 50. https://doi.org/10.1111/and.12880
Naujokas MF, Anderson B, Ahsan H et al (2013) The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect 121:295–302. https://doi.org/10.1289/ehp.1205875
Davey JC, Bodwell JE, Gosse JA et al (2007) Arsenic as an endocrine disruptor: effects of arsenic on estrogen receptor-mediated gene expression in vivo and in cell culture. Toxicol Sci 98:75–86. https://doi.org/10.1093/toxsci/kfm013
Zargari F, Rahaman MS, KazemPour R et al (2022) Arsenic, oxidative stress and reproductive system. J Xenobiot 12:214–222. https://doi.org/10.3390/jox12030016
Acknowledgements
The authors would like to thank the Research Center of Basic Medical School of Guizhou Medical University for providing experimental equipment.
Funding
This work was supported by the Guizhou Provincial Education Department Youth Science and Technology Talent Growth Project [grant No. Qianjiaohe KY, (2021) 150] and the Science and Technology Project of Guizhou Province [grant No. Qiankehejichu-ZK (2021) Yiban 374].
Author information
Authors and Affiliations
Contributions
Qi Wang and Yanping Yang: conceived and designed the experiment. Yan Hong, Jing Han, Zhe Yang, Nanmin Huang, and Binwei Xu: performed the experiments and data analysis. Qi Wang and Yanping Yang: prepared figures and the manuscript. All authors contributed to the article and approved the submitted version. Qi Wang and Yanping Yang confirmed the authenticity of the raw data.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Hong, Y., Han, J. et al. D-Limonene Alleviates Oxidative Stress Injury of the Testis Induced by Arsenic in Rat. Biol Trace Elem Res 202, 2776–2785 (2024). https://doi.org/10.1007/s12011-023-03881-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03881-z