Abstract
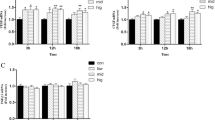
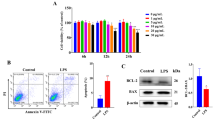
Endometritis is a common postpartum disease of female animals that causes significant losses to the goat industry. High levels of cortisol induced by various stresses after delivery severely inhibit innate immunity and tissue repair. The repair ability of the endometrium is closely related to the reproductive performance of goats. Selenium (Se) is an essential trace element in animals that has powerful antioxidant and immunity-enhancing functions. In this study, we established a goat model of endometritis at high cortisol (Hydrocortisone) levels to investigate the effect of Se (supplement additive) on endometrial repair. The results showed that the clinical symptoms, %PMN in uterine secretions, morphological endometrial damage, and the gene expression of BAX were reduced in the goats with Se supplementation compared with those in the model group. Se increased the gene expression of BCL2, VEGFA, TGFB1, and PCNA and activated the PI3K/AKT and Wnt/β-catenin signaling pathways in goats with Se supplementation. In conclusion, Se reduced the inflammatory response, increased the proliferation, and decreased the apoptosis of endometrial cells to promote endometrial tissue repair in goats with endometritis at high cortisol levels. It probably achieved this effect of promoting repair by activating the Wnt/β-catenin and PI3K/AKT pathways and affecting the gene expression of VEGFA, TGFB1, PCNA, BCL2, and BAX.









Similar content being viewed by others
Data Availability
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
References
Dong J, Li J, Li J, Cui L, Meng X, Qu Y et al (2019) The proliferative effect of cortisol on bovine endometrial epithelial cells. Reprod Biol Endocrinol 17(1):97. https://doi.org/10.1186/s12958-019-0544-1
Gilbert RO, Shin ST, Guard CL, Erb HN, Frajblat M (2005) Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 64(9):1879–1888. https://doi.org/10.1016/j.theriogenology.2005.04.022
Sheldon IM, Molinari PCC, Ormsby TJR, Bromfield JJ (2020) Preventing postpartum uterine disease in dairy cattle depends on avoiding, tolerating and resisting pathogenic bacteria. Theriogenology 150:158–165. https://doi.org/10.1016/j.theriogenology.2020.01.017
de Boer M, Buddle BM, Heuer C, Hussein H, Zheng T, LeBlanc SJ et al (2015) Associations between intrauterine bacterial infection, reproductive tract inflammation, and reproductive performance in pasture-based dairy cows. Theriogenology 83(9):1514–1524. https://doi.org/10.1016/j.theriogenology.2015.01.032
Okawa H, Fujikura A, Wijayagunawardane MMP, Vos P, Taniguchi M, Takagi M (2017) Effect of diagnosis and treatment of clinical endometritis based on vaginal discharge score grading system in postpartum Holstein cows. J Vet Med Sci 79(9):1545–1551. https://doi.org/10.1292/jvms.16-0593
Lu W, Xu ZM, Liu Q, Yu NN, Yu JB, Li WL et al (2021) Inhibitory effect of bovine adipose-derived mesenchymal stem cells on lipopolysaccharide induced inflammation of endometrial epithelial cells in dairy cows. Front Vet Sci 8:726328. https://doi.org/10.3389/fvets.2021.726328
Bruna A, Nicolas M, Munoz A, Kyriakis JM, Caelles C (2003) Glucocorticoid receptor-JNK interaction mediates inhibition of the JNK pathway by glucocorticoids. EMBO J 22(22):6035–6044. https://doi.org/10.1093/emboj/cdg590
Tzora A, Leontides LS, Amiridis GS, Manos G, Fthenakis GC (2002) Bacteriological and epidemiological findings during examination of the uterine content of ewes with retention of fetal membranes. Theriogenology 57(7):1809–1817. https://doi.org/10.1016/s0093-691x(02)00684-2
Singh N, Rajya BS (1977) Pathology of female reproductive system in goats. Indian J Anim Sci 47(1):22–28
Sokkar SM, Kubba MA, Al-Augaidy F (1980) Studies on natural and experimental endometritis in ewes. Vet Pathol 17(6):693–698. https://doi.org/10.1177/030098588001700606
Sheldon IM, Williams EJ, Miller AN, Nash DM, Herath S (2008) Uterine diseases in cattle after parturition. Vet J 176(1):115–121. https://doi.org/10.1016/j.tvjl.2007.12.031
Williams EJ, Fischer DP, Noakes DE, England GC, Rycroft A, Dobson H et al (2007) The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 68(4):549–559. https://doi.org/10.1016/j.theriogenology.2007.04.056
Cui L, Zheng Y, Wang H, Dong J, Li J, Song Q et al (2020) Cortisol inhibits the Escherichia coli-induced endometrial inflammatory response through NF-kappaB and MAPK pathways in postpartum goats. Anim Reprod Sci 215:106333. https://doi.org/10.1016/j.anireprosci.2020.106333
Shao CY, Wang H, Meng X, Zhu JQ, Wu YQ, Li JJ (2012) Characterization of the innate immune response in goats after intrauterine infusion of E. coli using histopathological, cytologic and molecular analyses. Theriogenology 78(3):593–604. https://doi.org/10.1016/j.theriogenology.2012.03.005
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21(1):55–89. https://doi.org/10.1210/edrv.21.1.0389
Probo M, Cairoli F, Kindahl H, Faustini M, Galeati G, Veronesi MC (2011) Peripartal hormonal changes in Alpine goats: a comparison between physiological and pathological parturition. Reproduction in domestic animals =. Zuchthygiene 46(6):1004–1010. https://doi.org/10.1111/j.1439-0531.2011.01775.x
Zoldan K, Moellmer T, Schneider J, Fueldner C, Knauer J, Lehmann J (2014) Increase of CD25 expression on bovine neutrophils correlates with disease severity in post-partum and early lactating dairy cows. Dev Comp Immunol 47(2):254–263. https://doi.org/10.1016/j.dci.2014.08.002
Alabdullah HA, Fox LK, Gay JM, Barrington GM (2018) Interactive effects of dexamethasone and opsonized Mycoplasma bovis on bovine neutrophil function in vitro. Vet Immunol Immunopathol 196:18–21. https://doi.org/10.1016/j.vetimm.2017.12.008
Amsterdam A, Tajima K, Sasson R (2002) Cell-specific regulation of apoptosis by glucocorticoids: implication to their anti-inflammatory action. Biochem Pharmacol 64(5-6):843–850. https://doi.org/10.1016/s0006-2952(02)01147-4
Kieliszek M, Blazejak S, Bzducha-Wrobel A, Kurcz A (2016) Effects of selenium on morphological changes in Candida utilis ATCC 9950 yeast cells. Biol Trace Elem Res 169(2):387–393. https://doi.org/10.1007/s12011-015-0415-3
Gashu D, Nalivata PC, Amede T, Ander EL, Bailey EH, Botoman L et al (2021) The nutritional quality of cereals varies geospatially in Ethiopia and Malawi. Nature 594(7861):71–76. https://doi.org/10.1038/s41586-021-03559-3
Allison RD, Laven RA (2000) Effect of vitamin E supplementation on the health and fertility of dairy cows: a review. Vet Rec 147(25):703–708
Li M, Cheng W, Zhang L (2021) Maternal selenium deficiency suppresses proliferation, induces autophagy dysfunction and apoptosis in the placenta of mice. Metallomics 13(11):mfab058. https://doi.org/10.1093/mtomcs/mfab058
Shi L, Yue W, Zhang C, Ren Y, Zhu X, Wang Q et al (2010) Effects of maternal and dietary selenium (Se-enriched yeast) on oxidative status in testis and apoptosis of germ cells during spermatogenesis of their offspring in goats. Anim Reprod Sci 119(3-4):212–218. https://doi.org/10.1016/j.anireprosci.2010.02.012
Hu P, Zuo Z, Wang F, Peng X, Guan K, Li H et al (2018) The protective role of selenium in AFB1-induced tissue damage and cell cycle arrest in chicken’s bursa of Fabricius. Biol Trace Elem Res 185(2):486–496. https://doi.org/10.1007/s12011-018-1273-6
Samo SP, Malhi M, Kachiwal AB, Gadahi JA, Parveen F, Kalhoro NH et al (2020) Supranutritional selenium level minimizes high concentrate diet-induced epithelial injury by alleviating oxidative stress and apoptosis in colon of goat. BMC Vet Res 16(1):462. https://doi.org/10.1186/s12917-020-02653-4
Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69(Suppl 3):4–10. https://doi.org/10.1159/000088478
Kajdaniuk D, Marek B, Borgiel-Marek H, Kos-Kudła B (2013) Transforming growth factor β1 (TGFβ1) in physiology and pathology. Endokrynol Pol 64(5):384–396. https://doi.org/10.5603/ep.2013.0022
González-Magaña A, Blanco FJ (2020) Human PCNA structure, function and interactions. Biomolecules 10(4):570. https://doi.org/10.3390/biom10040570
Horsfall AJ, Abell AD, Bruning JB (2020) Targeting PCNA with peptide mimetics for therapeutic purposes. Chembiochem 21(4):442–450. https://doi.org/10.1002/cbic.201900275
Vaskivuo TE, Stenbäck F, Tapanainen JS (2002) Apoptosis and apoptosis-related factors Bcl-2, Bax, tumor necrosis factor-alpha, and NF-kappaB in human endometrial hyperplasia and carcinoma. Cancer 95(7):1463–1471. https://doi.org/10.1002/cncr.10876
Apte SS, Mattei MG, Olsen BR (1995) Mapping of the human BAX gene to chromosome 19q13.3-q13.4 and isolation of a novel alternatively spliced transcript, BAX delta. Genomics 26(3):592–594. https://doi.org/10.1016/0888-7543(95)80180-t
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74(4):609–619. https://doi.org/10.1016/0092-8674(93)90509-o
Shi X, Ran L, Liu Y, Zhong SH, Zhou PP, Liao MX et al (2018) Knockdown of hnRNP A2/B1 inhibits cell proliferation, invasion and cell cycle triggering apoptosis in cervical cancer via PI3K/AKT signaling pathway. Oncol Rep 39(3):939–950. https://doi.org/10.3892/or.2018.6195
Liu X, Zhang L, Liu Y, Cui J, Che S, An X et al (2018) Circ-8073 regulates CEP55 by sponging miR-449a to promote caprine endometrial epithelial cells proliferation via the PI3K/AKT/mTOR pathway. Biochim Biophys Acta Mol Cell Res 1865(8):1130–1147. https://doi.org/10.1016/j.bbamcr.2018.05.011
Kim H, Chung H, Kim HJ, Lee JY, Oh MY, Kim Y et al (2008) Id-1 regulates Bcl-2 and Bax expression through p53 and NF-kappaB in MCF-7 breast cancer cells. Breast Cancer Res Treat 112(2):287–296. https://doi.org/10.1007/s10549-007-9871-6
Hayashi K, Yoshioka S, Reardon SN, Rucker EB 3rd, Spencer TE, DeMayo FJ et al (2011) WNTs in the neonatal mouse uterus: potential regulation of endometrial gland development. Biol Reprod 84(2):308–319. https://doi.org/10.1095/biolreprod.110.088161
Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D et al (2011) WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB J 25(4):1176–1187. https://doi.org/10.1096/fj.10-175349
Miller C, Sassoon DA (1998) Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 125(16):3201–3211. https://doi.org/10.1242/dev.125.16.3201
Clevers H, Nusse R (2012) Wnt/β-catenin signaling and disease. Cell 149(6):1192–1205. https://doi.org/10.1016/j.cell.2012.05.012
Hatsell S, Rowlands T, Hiremath M, Cowin P (2003) Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia 8(2):145–158. https://doi.org/10.1023/a:1025944723047
Pooja T, Karunagaran D (2014) Emodin suppresses Wnt signaling in human colorectal cancer cells SW480 and SW620. Eur J Pharmacol 742:55–64. https://doi.org/10.1016/j.ejphar.2014.08.028
Olmeda D, Castel S, Vilaró S, Cano A (2003) Beta-catenin regulation during the cell cycle: implications in G2/M and apoptosis. Mol Biol Cell 14(7):2844–2860. https://doi.org/10.1091/mbc.e03-01-0865
Strzalka W, Ziemienowicz A (2011) Proliferating cell nuclear antigen (PCNA): a key factor in DNA replication and cell cycle regulation. Ann Bot 107(7):1127–1140. https://doi.org/10.1093/aob/mcq243
Zhang L, Cai Y, Li L, Chen C, Zhao H, Zhang Z et al (2022) Effects of luteolin on biofilm of Trueperella pyogenes and its therapeutic effect on rat endometritis. Int J Mol Sci 23(22):14451. https://doi.org/10.3390/ijms232214451
Zhao QX, Chen YW, Belzile N, Wang M (2010) Low volume microwave digestion and direct determination of selenium in biological samples by hydride generation-atomic fluorescence spectrometry. Anal Chim Acta 665(2):123–128. https://doi.org/10.1016/j.aca.2010.03.040
Buczkowska J, Kozdrowski R, Nowak M, Ras A, Staroniewicz Z, Siemieniuch MJ (2014) Comparison of the biopsy and cytobrush techniques for diagnosis of subclinical endometritis in mares. Reprod Biol Endocrinol 12:27. https://doi.org/10.1186/1477-7827-12-27
Li B, Zhang Q, Sun J, Lai D (2019) Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Res Ther 10(1):257. https://doi.org/10.1186/s13287-019-1368-9
Yao Y, Chen R, Wang G, Zhang Y, Liu F (2019) Exosomes derived from mesenchymal stem cells reverse EMT via TGF-β1/Smad pathway and promote repair of damaged endometrium. Stem Cell Res Ther 10(1):225. https://doi.org/10.1186/s13287-019-1332-8
Zhu Y, Tang Y, Fan Y, Wu D (2022) MiR-196a-5p facilitates progression of estrogen-dependent endometrial cancer by regulating FOXO1. Histol Histopathol:18572. https://doi.org/10.14670/hh-18-572
Song P, Liu C, Sun M, Liu J, Lin P, Wang A et al (2022) Oxidative stress induces bovine endometrial epithelial cell damage through mitochondria-dependent pathways. Animals 12(18):2444. https://doi.org/10.3390/ani12182444
Cicinelli E, Vitagliano A, Loizzi V, De Ziegler D, Fanelli M, Bettocchi S et al (2021) Altered gene expression encoding cytochines, grow factors and cell cycle regulators in the endometrium of women with chronic endometritis. Diagnostics 11(3):471. https://doi.org/10.3390/diagnostics11030471
Collery P, Veena V, Harikrishnan A, Desmaele D (2019) The rhenium(I)-diselenoether anticancer drug targets ROS, TGF-β1, VEGF-A, and IGF-1 in an in vitro experimental model of triple-negative breast cancers. Invest New Drugs 37(5):973–983. https://doi.org/10.1007/s10637-019-00727-1
Chi X, Liu Z, Wei W, Hu X, Wang Y, Wang H et al (2021) Selenium-rich royal jelly inhibits hepatocellular carcinoma through PI3K/AKT and VEGF pathways in H22 tumor-bearing mice. Food Funct 12(19):9111–9127. https://doi.org/10.1039/d1fo01070k
Hamid M, Abdulrahim Y, Liu D, Qian G, Khan A, Huang K (2018) The hepatoprotective effect of selenium-enriched yeast and gum arabic combination on carbon tetrachloride-induced chronic liver injury in rats. J Food Sci 83(2):525–534. https://doi.org/10.1111/1750-3841.14030
Yu JS, Cui W (2016) Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development 143(17):3050–3060. https://doi.org/10.1242/dev.137075
Xu XY, Zhang J, Qi YH, Kong M, Liu SA, Hu JJ (2018) Linc-ROR promotes endometrial cell proliferation by activating the PI3K-Akt pathway. Eur Rev Med Pharmacol Sci 22(8):2218–2225. https://doi.org/10.26355/eurrev_201804_14807
Yao X, Ei-Samahy MA, Fan L, Zheng L, Jin Y, Pang J et al (2018) In vitro influence of selenium on the proliferation of and steroidogenesis in goat luteinized granulosa cells. Theriogenology 114:70–80. https://doi.org/10.1016/j.theriogenology.2018.03.014
Liu D, Lin J, He W, Huang K (2021) Selenium and taurine combination is better than alone in protecting lipopolysaccharide-induced mammary inflammatory lesions via activating PI3K/Akt/mTOR signaling pathway by scavenging intracellular ROS. Oxid Med Cell Longev 2021:5048375. https://doi.org/10.1155/2021/5048375
Kiewisz J, Wasniewski T, Kmiec Z (2015) Participation of WNT and β-catenin in physiological and pathological endometrial changes: association with angiogenesis. Biomed Res Int 2015:854056. https://doi.org/10.1155/2015/854056
Zhang F, Zhang J, Li J, Yan P, Li Y, Zhang Y et al (2022) Effect of VD3 on cell proliferation and the Wnt signaling pathway in bovine endometrial epithelial cells treated with lipopolysaccharide. Theriogenology 193:68–76. https://doi.org/10.1016/j.theriogenology.2022.09.002
Sharma AR, Sharma G, Lee YH, Chakraborty C, Lee SS, Seo EM (2022) Sodium selenite promotes osteoblast differentiation via the WNT/ß-catenin signaling pathway. Cell J 24(6):309–315. https://doi.org/10.22074/cellj.2022.8314
Liu X, Mao Y, Huang S, Li W, Zhang W, An J et al (2022) Selenium nanoparticles derived from Proteus mirabilis YC801 alleviate oxidative stress and inflammatory response to promote nerve repair in rats with spinal cord injury. Regen Biomater 9:rbac042. https://doi.org/10.1093/rb/rbac042
Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y et al (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108(6):837–847. https://doi.org/10.1016/s0092-8674(02)00685-2
Cohen P, Frame S (2001) The renaissance of GSK3. Nat Rev Mol Cell Biol 2(10):769–776. https://doi.org/10.1038/35096075
Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378(6559):785–789. https://doi.org/10.1038/378785a0
Zheng R, Zhang ZH, Chen C, Chen Y, Jia SZ, Liu Q et al (2017) Selenomethionine promoted hippocampal neurogenesis via the PI3K-Akt-GSK3β-Wnt pathway in a mouse model of Alzheimer’s disease. Biochem Biophys Res Commun 485(1):6–15. https://doi.org/10.1016/j.bbrc.2017.01.069
Redd MJ, Cooper L, Wood W, Stramer B, Martin P (2004) Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci 359(1445):777–784. https://doi.org/10.1098/rstb.2004.1466
Karin M, Clevers H (2016) Reparative inflammation takes charge of tissue regeneration. Nature 529(7586):307–315. https://doi.org/10.1038/nature17039
Sweet M, Mansell A (2017) The nicer side of innate immunity: wound healing and tissue regeneration. Semin Cell Dev Biol 61:1–2. https://doi.org/10.1016/j.semcdb.2016.12.006
Cao L, Gao S, Liu J, Wang J, Qin R (2023) Selenomethionine protects against Escherichia coli-induced endometritis by inhibiting inflammation and necroptosis via regulating the PPAR-γ/NF-κB pathway. Chem Biol Interact 379:110532. https://doi.org/10.1016/j.cbi.2023.110532
Zhang Z, Gao X, Cao Y, Jiang H, Wang T, Song X et al (2015) Selenium deficiency facilitates inflammation through the regulation of TLR4 and TLR4-related signaling pathways in the mice uterus. Inflammation 38(3):1347–1356. https://doi.org/10.1007/s10753-014-0106-9
Funding
This work was supported by the National Natural Science Foundation of China (32072937, 32102735), the Natural Science Foundation of Jiangsu Province (BK20210808), Postgraduate Research & Practice Innovation Program of Jiangsu Province (Yangzhou University) (SJCX21_1637), the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2022]499), the 333 High-level Talent Training Project of Jiangsu Province (CN), the 111 Project (D18007), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
HL: conceptualization, writing – original draft. CY: methodology. HW: supervision. LC, KL, and LG: formal analysis. JD: writing – review and editing, funding acquisition, supervision. JL: funding acquisition, supervision.
Corresponding authors
Ethics declarations
Ethics Approval
All experimental procedures were approved by the Animal Care and Use Committee of Yangzhou University (approval ID: 20190303). The animal experimental procedures conducted were in accordance with the Guide for Care and Use of Agricultural Animals of Yangzhou University.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Yuan, C., Wang, H. et al. The Effect of Selenium on Endometrial Repair in Goats with Endometritis at High Cortisol Levels. Biol Trace Elem Res 202, 2564–2576 (2024). https://doi.org/10.1007/s12011-023-03866-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03866-y